
��ͼ��ֱ�߽��㴦��ԲȦΪNaCl������![]() ���ӻ�
���ӻ�![]() ����������λ�ã������������ڿռ�3�����ഹֱ�ķ����϶��ǵȾ������еģ�
����������λ�ã������������ڿռ�3�����ഹֱ�ķ����϶��ǵȾ������еģ�
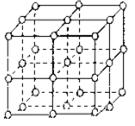
(1)�뽫���д���![]() ���ӵ�ԲȦͿ��(���ؿ��������С)�������NaCl����ṹʾ��ͼ��
���ӵ�ԲȦͿ��(���ؿ��������С)�������NaCl����ṹʾ��ͼ��
(2)�����У���ÿ��![]() ���ӵ���Χ������ӽ����Ҿ�����ȵ�
���ӵ���Χ������ӽ����Ҿ�����ȵ�![]() ����_________����
����_________����
(3)������ÿһ���ظ��Ľṹ��Ԫ�о�������NaCl��������������Ķ����ϡ����ϡ����ϵ�![]() ��Cl-Ϊ�þ����������ڵľ��������У�һ��������Cl-���ӵĸ�������_____________����(�����ʽ)___________��
��Cl-Ϊ�þ����������ڵľ��������У�һ��������Cl-���ӵĸ�������_____________����(�����ʽ)___________��![]() ���ӵĸ�������___________����(�����ʽ)___________��
���ӵĸ�������___________����(�����ʽ)___________��
(4)��NaCl��Ħ������Ϊ![]() ��ʳ�ξ�����ܶ�Ϊ
��ʳ�ξ�����ܶ�Ϊ![]() �������ӵ�����Ϊ
�������ӵ�����Ϊ![]() ��ʳ�ξ���������������������������ļ�ľ���Ϊ___________cm��
��ʳ�ξ���������������������������ļ�ľ���Ϊ___________cm��
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��֪ʶ����������ѵ������������ѧ ���ͣ�043
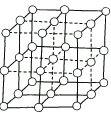
ͼ��ֱ�߽��㴦��ԲȦΪNaCl������Na+��Cl��������λ�ã��뽫���д���Na+��ԲȦͿ��(����������Ĵ�С)�������NaCl����Ľṹʾ��ͼ��������ÿ��Na+��Χ��������ҵȾ����Na+��________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
(1)�뽫���д���Na+���ӵ�ԲȦͿ��(���ؿ��������С)�������NaCl����ṹʾ��ͼ��
(2)�����У���ÿ��Na+���ӵ���Χ������ӽ����Ҿ�����ȵ�Na+����________����
(3)������ÿһ���ظ��Ľṹ��Ԫ�о�������NaCl��������������Ķ����ϣ����ϣ����ϵ�Na+��Cl��Ϊ�þ����������ڵľ��������У�һ�������У�Cl�����ӵĸ�������________����________(�����ʽ)��Na+���ӵĸ�������________����________(�����ʽ)��
(4)��NaCl��Ħ������ΪMg��mol��ʳ�ξ�����ܶ�Ϊpg��cm3������٤������Ϊ![]() ��ʳ�ξ���������������������������ļ�ľ���Ϊ________cm��
��ʳ�ξ���������������������������ļ�ľ���Ϊ________cm��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007�������һ��ͳһ�����⡢��ѧ ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ֱ�߽��㴦��ԲȦΪNaCl������Na+��Cl-������λ�á������������ڿռ��������ഹֱ�ķ����϶��ǵȾ������еġ�
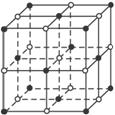
(1)�뽫���д���Na+��ԲȦͿ�ڣ������������С���������NaCl����ṹʾ��ͼ��
(2)�����У���ÿ��Na+��Χ������ӽ����Ҿ�����ȵ�Na+���У� ��
A.4�� B.6�� C.8�� D.12��
(3)�����У���ÿ��Na+��������Ҿ��������n��Cl-��Χ�ɵĿռ伸�ι���Ϊ�� ��
A.�������� B.��������
C.�������� D.����ʮ����
(4)������ÿһ���ظ��Ľṹ��Ԫ�о�������NaCl��������������Ķ����ϡ����ϡ����ϵ�Na+��Cl-Ϊ�þ����������ڵľ��������У�һ��������Cl-�ĸ�������________������________�������ʽ����Na+�ĸ�������___________����________________________________�������ʽ����
(5)��NaCl��Ħ������ΪM g��mol-1��ʳ�ξ�����ܶ�Ϊ�� g��cm-3�������ӵ�����ΪNA��ʳ�ξ������������������Na+���ĵľ���Ϊ___________cm��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com