
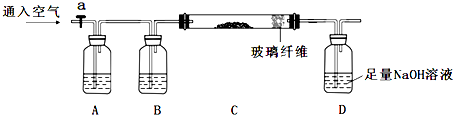
 ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨ�������Ƶ���������ԼΪ82%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol•L-1��������еζ�������������⣺
ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨ�������Ƶ���������ԼΪ82%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol•L-1��������еζ�������������⣺���� ��2������Ӧ����ʽ�ζ�����ȡ��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܻ���Һ�ܣ��ü�����ָʾ��ʱ���ζ�ǰ�����������ƣ��ζ��������ɫ��ֹͣ�ζ���
��4��c��HCl��=0.2mol/L��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�������c��NaOH������һ������500mL��Һ��NaOH�����ʵ�����������Ʒ������5.0g��������������
��5������c�����⣩=$\frac{V��������c������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��6��A��C�̶ȼ����1mL��˵��ÿ����С��֮����0.1mL��A���Ŀ̶�Ϊ25.00mL���ݴ�ȷ��B�Ŀ̶ȣ�ע��ζ��ܵ�������ֵС��������ֵ�ζ��ܻ����·�����Ƥ���̶ȣ�
��� �⣺��2������Ӧ����ʽ�ζ�����ȡ���ʴ�Ϊ����ʽ��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܣ�������ƿ�У��÷�̪��ָʾ��ʱ���ζ�ǰ����̪�����죬����ζ��յ�ʱ�۲쵽��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ����ʽ�ζ��ܡ���ƿ����ɫ���ޣ�
��4��c��HCl��=0.2mol•L-1��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�����֪c��NaOH��=0.2mol/L��
��500mL��Һ��NaOH�����ʵ���Ϊ0.5L��0.2mol/L=0.1mol����NaOH������Ϊ0.1mol��40g/mol=4.0g������5.0g��Ʒ��NaOH����������Ϊ$\frac{4.0g}{5.0g}$��100%=80.0%��
�ʴ�Ϊ��80.0%��
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ������٣�����V���ᣩ���٣�����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�ͣ����������Ƶ���������ƫ�ͣ���A��ȷ��
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫ�ͣ�����V���ᣩƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�����������Ƶ���������ƫ�ߣ���B����
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦����NaOH�����ʵ������٣�����V���ᣩ���٣�����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�ͣ����������Ƶ���������ƫ�ͣ���C��ȷ��
D���ζ����յ�ʱ���ζ��ܼ�������Һ�Σ�����V���ᣩƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�����������Ƶ���������ƫ�ߣ���D����
E������ʽ�ζ��ܿ�ʼʱ���ӣ�����ƫ�����յ�ʱ���ӣ�����ƫС������֮��ƫС������V���ᣩ���٣�����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�ͣ����������Ƶ���������ƫ�ͣ���E��ȷ��
�ʴ�Ϊ��ACE��
��6��A��C�̶ȼ����1mL��˵��ÿ����С��֮����0.10mL��A���Ŀ̶�Ϊ25.00mL��A��B֮�����ĸ�С���������0.40mL����B��25.40mL���ζ��̶ܿ�ֵ���ϵ��¿̶����������ڵζ��ܻ����·�����Ƥ���̶ȣ�50mL�ζ�����ʵ��ʢ��Һ����������50mL�����Һ�洦�Ķ�����a����ζ�����Һ���������ڣ�50-a��mL��
�ʴ�Ϊ��25.40�����ڣ�
���� ���⿼���к͵ζ������յζ�ԭ����ʵ��������������Ϊ���Ĺؼ���ע�����������ü��㹫ʽ���ᡢ����������Ŀ�ѶȲ���


 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
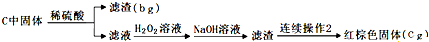
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
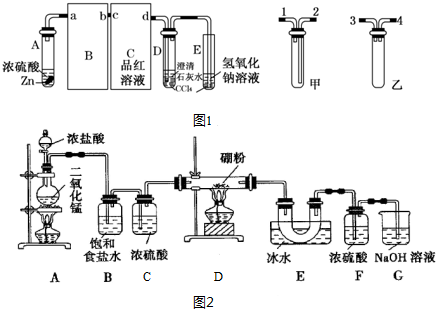
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
| ����KMnO4���/mL | 19.98 | 20.02 | 20.20 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼ | B�� | �� | C�� | �� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H2 | B�� | BeCl2 | C�� | CO2 | D�� | HClO |
�鿴�𰸺ͽ���>>
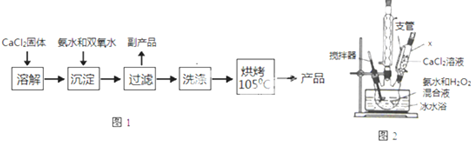
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
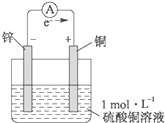 ��ͼΪп��ͭ������ͭ��Һ���ɵ�ԭ��أ���д���������Լ�����ܷ�Ӧ��
��ͼΪп��ͭ������ͭ��Һ���ɵ�ԭ��أ���д���������Լ�����ܷ�Ӧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com