
 ӣ
ӣ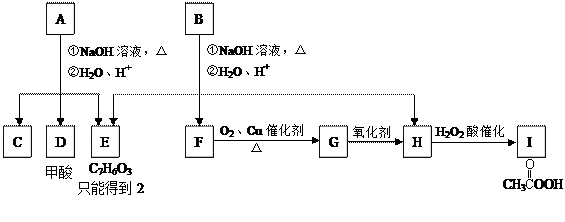

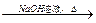 CH3COONa£«
CH3COONa£« £ONa£©
£ONa£© ĢōÕ½100µ„ŌŖ¼ģ²āŹŌ¾ķĻµĮŠ“š°ø
ĢōÕ½100µ„ŌŖ¼ģ²āŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
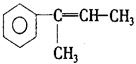 ijÖÖŅ»ĀČ“śĪļ“ęŌŚĖ³·“Ņģ¹¹£¬Š“³öĘäÖŠæÕ¼ä½į¹¹ĪŖ”°·“Ź½”±µÄ½į¹¹¼ņŹ½ ”£
ijÖÖŅ»ĀČ“śĪļ“ęŌŚĖ³·“Ņģ¹¹£¬Š“³öĘäÖŠæÕ¼ä½į¹¹ĪŖ”°·“Ź½”±µÄ½į¹¹¼ņŹ½ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 20·Ö£©
20·Ö£© µÄÓŠ»śĪļµÄ½į¹¹¼ņŹ½
µÄÓŠ»śĪļµÄ½į¹¹¼ņŹ½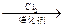
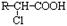 £»
£»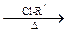 R-O-R”ä (R-”¢R”ä-“ś±ķĢž»ł)”£
R-O-R”ä (R-”¢R”ä-“ś±ķĢž»ł)”£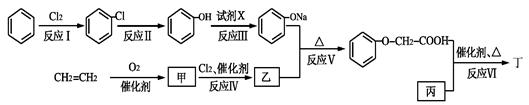
 £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
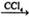 CH3CHBrCH2Br ¢ŚCH3CH2OH
CH3CHBrCH2Br ¢ŚCH3CH2OH 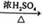 CH2=CH2+H2O
CH2=CH2+H2O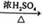 CH3COOCH2CH3+H2O¢ÜC6H6+HNO3
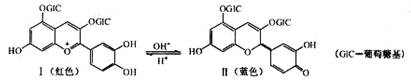
CH3COOCH2CH3+H2O¢ÜC6H6+HNO3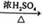 C6H5NO2+H2O
C6H5NO2+H2O| A£®¢Ł¢Ś | B£®¢Ū¢Ü | C£®¢Ł¢Ū | D£®¢Ś¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®“æ¾»µÄ±½·ÓŹĒ·ŪŗģÉ«¾§Ģå |
| B£®ÓŠĢŲŹāĘųĪ¶ |
| C£®Ņ×ČÜÓŚŅŅ“¼”¢ŅŅĆѵČÓŠ»śČܼĮ£¬²»Ņ×ČÜÓŚĄäĖ® |
| D£®±½·ÓÓŠ¶¾£¬Õ“µ½Ę¤·ōÉĻ£¬æÉÓĆÅØNaOHČÜŅŗĻ“µÓ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®»ØĒąÜÕæÉ×÷ĪŖŅ»ÖÖĖį¼īÖøŹ¾¼Į | B£®IŗĶ¢ņÖŠ¾łŗ¬ÓŠ¶žøö±½»· |
| C£®IŗĶ¢ņÖŠ³żĮĖĘĻĢŃĢĒ»łĶā£¬ĖłÓŠĢ¼Ō×ÓæÉÄܹ²Ę½Ćę | |
| D£®IŗĶ¢ņ¾łÄÜÓėFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

 ¢ŁÓĆĖ®¼ų±š±½”¢ŅŅ“¼”¢äå±½
¢ŁÓĆĖ®¼ų±š±½”¢ŅŅ“¼”¢äå±½ ¢ŚŅŅĻ©ŗĶ¼×ĶéæÉÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ų±š
¢ŚŅŅĻ©ŗĶ¼×ĶéæÉÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ų±š ¢ŪĪŖ³żČ„±½ÖŠµÄÉŁĮæ±½·Ó,Ļņ»ģŗĻĪļÖŠ¼ÓČėŹŹĮæµÄäåĖ®ŗó¹żĀĖ
¢ŪĪŖ³żČ„±½ÖŠµÄÉŁĮæ±½·Ó,Ļņ»ģŗĻĪļÖŠ¼ÓČėŹŹĮæµÄäåĖ®ŗó¹żĀĖ
| A£®¢Ł¢Ś | B£®¢Ł¢Ū | C£®¢Ś¢Ū | D£®¢Ł¢Ś¢Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ś | B£®¢Ś¢Ū | C£®¢Ū¢Ü | D£®¢Ś¢Ü |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com