
��12�֣�Ϊ�������鶨�顷2012���һ��ŵ�ڵ��ں����������������⣬��������������˲�ͬ��Ŭ������������Դ�Ŀ��������ã�CH3OH��������������ǵ���Ұ��Խ��Խ�ܵ����ǵĹ�ע��
��1����ͼ����CO��g��+2H2��g����CH3OH��g�����й����е������仯���ߡ�����a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾ���������˵����ȷ���ǣ� ��
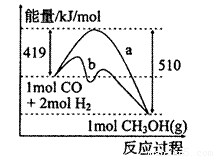
A���÷�Ӧ�����ȷ�Ӧ
B��ʹ�ô�����Ӧ�ȼ�С
C���Ȼ�ѧ����ʽΪCO��g��+2H2��g�� CH3OH��g�� ��H=-510kJ��mol
��2���ɣ�1���ƶϣ�CO��g��+2H2��g�� CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
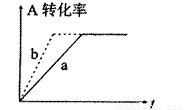
A������CO��Ũ�� B����С�������ݻ�
C��������� D�������¶�
��3���ɼ״��������Լ�ǿ�����������Һ������ȼ�ϵ�أ��������ƹ�ʹ�ã��ٶ��ŵ�����У��״���ȫ��������������̼�������������CO32-���õ�صĸ�����Ӧ�����ӷ���ʽΪ ���ŵ�����е������Һ��pH�� ����½����������������䡱��������16�˼״�����ȫ�����������ܣ������øù������ͷŵĵ��ܵ������������ͭ��Һ������������������Ϊ80������õ����������ʵ����� ��
��4��ijͬѧ���״���ȫȼ������CO2����ͨ��200mL 0��1 mol/L��ʯ��ˮ�õ�lg��������ôͨ���CO2���������Ϊ����̬�� ��
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡʡ����У2012�������ѧ�ڵ��Ĵ�������ѧ���� ���ͣ�022
Ϊ�������鶨�顷2012���һ��ŵ�ڵ��ں����������������⣬��������������˲�ͬ��Ŭ������������Դ�Ŀ��������ã�CH3OH��������������ǵ���Ұ��Խ��Խ�ܵ����ǵĹ�ע��
(1)��ͼ����CO(g)��2H2(g)��CH3OH(g)���й����е������仯���ߣ�����a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾ���������˵����ȷ����_______
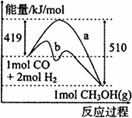
A���÷�Ӧ�����ȷ�Ӧ
B��ʹ�ô�����Ӧ�ȼ�С
C���Ȼ�ѧ����ʽΪCO(g)��2H2(g)![]() CH3OH(g)����H����510 kJ/mol
CH3OH(g)����H����510 kJ/mol
D���Ȼ�ѧ����ʽΪCO(g)��2H2(g)![]() CH3OH(g)����H����91 kJ/mol
CH3OH(g)����H����91 kJ/mol
(2)��(1)�ƶϣ�CO(g)��2H2(g)![]() CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̣���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ��_______
CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̣���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ��_______
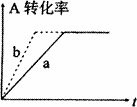
A������CO��Ũ��
B����С�������ݻ�
C���������
D�������¶�
(3)�ɼ״��������Լ�ǿ�����������Һ������ȼ�ϵ�أ��������ƹ�ʹ�ã��ٶ��ŵ�����У��״���ȫ��������������̼�������������CO32�����õ�صĸ�����Ӧ�����ӷ���ʽΪ_________���ŵ�����е������Һ��pH��_________(��½����������������䡱)������16�˼״�����ȫ�����������ܣ������øù������ͷŵĵ��ܵ������������ͭ��Һ������������������Ϊ80������õ����������ʵ�����_________��
(4)ijͬѧ���״���ȫȼ������CO2����ͨ��200 mL��0.1 mol/L��ʯ��ˮ�õ�1 g��������ôͨ���CO2���������Ϊ(��̬)_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2009���ٿ��ĸ籾���������������ϣ�����192�����ҵ�̸�д����ٿ���ᣬ���֡������鶨�顷���й�2012����2020���ȫ��CO2����Э�顣aͼΪ̼ѭ��ʾ��ͼ��bͼ��ʾ��̬ϵͳ���йسɷ�֮��Ĺ�ϵ�����ͼ�ش�
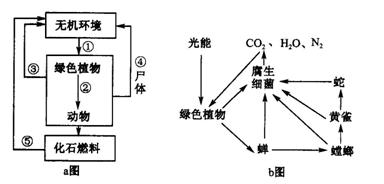
��1����aͼ��֪�����ʹ�����CO2��������Ҫ;���Ǽ��� ������ ����������ţ����ڢٺ͢ڵĹ�����̼�ֱ��� �� ����ʽ����������
��2��bͼ���ɲ�ʳ��ϵ���γɵ�ʳ������ ��������������� �������ߡ�
��3������ɫֲ�����̶���̫��������ΪW kJ����ͼ�����Ӫ��������õ��������Ϊ
kJ��
��4������bͼ��֪��̬ϵͳ���� �Ĺ��ܡ�
��5���۷������ͬ������棬����Ϊ���������������ǵļ�����Ȥ�����磺�۷��ڷ�����Դ��ͨ������ԲȦ�衱��β�衱��֪ͬ����Դ��λ�ã�������Ϣ���� ��Ϣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�Ϊ�������鶨�顷2012���һ��ŵ�ڵ��ں����������������⣬��������������˲�ͬ��Ŭ������������Դ�Ŀ��������ã�CH3OH��������������ǵ���Ұ��Խ��Խ�ܵ����ǵĹ�ע��
��1����ͼ����CO��g��+2H2��g����CH3OH��g�����й����е������仯���ߡ�����a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾ���������˵����ȷ���ǣ� ��
A���÷�Ӧ�����ȷ�Ӧ
B��ʹ�ô�����Ӧ�ȼ�С
C���Ȼ�ѧ����ʽΪCO��g��+2H2��g�� CH3OH��g�� ��H=-510kJ��mol
��2���ɣ�1���ƶϣ�CO��g��+2H2��g��![]() CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
A������CO��Ũ�� B����С�������ݻ�
C��������� D�������¶�
��3���ɼ״��������Լ�ǿ�����������Һ������ȼ�ϵ�أ��������ƹ�ʹ�ã��ٶ��ŵ�����У��״���ȫ��������������̼�������������CO32-���õ�صĸ�����Ӧ�����ӷ���ʽΪ ���ŵ�����е������Һ��pH�� ����½����������������䡱��������16�˼״�����ȫ�����������ܣ������øù������ͷŵĵ��ܵ������������ͭ��Һ������������������Ϊ80������õ����������ʵ����� ��
��4��ijͬѧ���״���ȫȼ������CO2����ͨ��200mL 0��1 mol/L��ʯ��ˮ�õ�lg��������ôͨ���CO2���������Ϊ����̬�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡʡ����У�������Ĵ�������ѧ�Ծ� ���ͣ���ѡ��
��12�֣�Ϊ�������鶨�顷2012���һ��ŵ�ڵ��ں����������������⣬��������������˲�ͬ��Ŭ������������Դ�Ŀ��������ã�CH3OH��������������ǵ���Ұ��Խ��Խ�ܵ����ǵĹ�ע��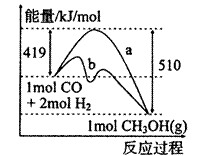
��1����ͼ����CO��g��+2H2��g����CH3OH��g�����й����е������仯���ߡ�����a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾ���������˵����ȷ���ǣ� ��
A���÷�Ӧ�����ȷ�Ӧ
B��ʹ�ô�����Ӧ�ȼ�С
C���Ȼ�ѧ����ʽΪCO��g��+2H2��g�� CH3OH��g����H=-510kJ��mol
��2���ɣ�1���ƶϣ�CO��g��+2H2��g�� CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��
CH3OH(g)���ܱ������н��У�ͼ������a����һ�������¸÷�Ӧ�Ĺ��̡���ʹa���߱�Ϊb���ߣ��ɲ�ȡ�Ĵ�ʩ�ǣ� ��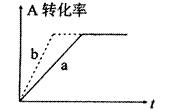
A������CO��Ũ�� B����С�������ݻ�
C��������� D�������¶�
��3���ɼ״��������Լ�ǿ�����������Һ������ȼ�ϵ�أ��������ƹ�ʹ�ã��ٶ��ŵ�����У��״���ȫ��������������̼�������������CO32-���õ�صĸ�����Ӧ�����ӷ���ʽΪ ���ŵ�����е������Һ��pH�� ����½����������������䡱��������16�˼״�����ȫ�����������ܣ������øù������ͷŵĵ��ܵ������������ͭ��Һ������������������Ϊ80������õ����������ʵ����� ��
��4��ijͬѧ���״���ȫȼ������CO2����ͨ��200mL 0��1 mol/L��ʯ��ˮ�õ�lg��������ôͨ���CO2���������Ϊ����̬�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com