
½«Ņ»¶ØÖŹĮæµÄMgŗĶAlµÄ»ģŗĻĪļĶ¶Čė2.0 mol/L£¬250 mLĻ”ĮņĖįÖŠ£¬¹ĢĢåČ«²æČܽā²¢²śÉśĘųĢ唣“ż·“Ó¦ĶźČ«ŗó£¬ĻņĖłµĆČÜŅŗÖŠ¼ÓČėŅ»¶ØÅØ¶ČµÄNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮæÓė¼ÓČėNaOHČÜŅŗµÄĢå»ż¹ŲĻµČēĶ¼ĖłŹ¾”£»Ų“šĻĀĮŠĪŹĢā£ŗ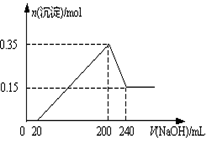
£Ø1£©ÓÉĶ¼æÉÖŖ¼ÓČėĆ¾µÄĪļÖŹµÄĮæŹĒ _____________________”£
£Ø2£©µ±¼ÓČėNaOHČÜŅŗµÄĢå»żŠ”ÓŚ20mLŹ±·¢ÉśµÄĄė×Ó·“Ó¦ŹĒ__________£¬µ±¼ÓČėNaOHČÜŅŗµÄĢå»ż“óÓŚ200 mLŹ±·¢ÉśµÄĄė×Ó·“Ó¦ŹĒ______________”£
£Ø3£©NaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ______________________”£
£Ø1£©0.15 mol
£Ø2£©H++OH”Ŗ= H2O Al(OH)3+ OH”Ŗ=AlO2”Ŗ +2 H2O”£
£Ø3£©5 mol/L»ņ5.0 mol/L
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŅņĪŖ×īŗóµÄ³Įµķ¶¼ĪŖĒāŃõ»ÆĆ¾ĪŖ0.15mol£¬ĖłŅŌ¼ÓČėĆ¾µÄĪļÖŹµÄĮæĪŖ0.15mol£»
£Ø2£©µ±ĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żŠ”ÓŚ20mLŹ±£¬ÖŠŗĶČÜŅŗÖŠµÄĒāĄė×Ó£¬ŅņĪŖ“ĖŹ±²¢ĪŽ³ĮµķÉś³É£¬µ±ĒāŃõ»ÆÄĘČÜŅŗµÄĢå»ż“óÓŚ200mLŗó£¬ĒāŃõøłĄė×Ó¾Ķ»įŗĶĒāŃõ»ÆĀĮ³Įµķ·¢Éś·“Ӧɜ³ÉĘ«ĀĮĖįĄė×Ó£»
£Ø3£©ÉčĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæÅضČĪŖX£¬ÓŠYmolµÄĀĮ£¬ŌņÓŠ£ŗ
0.15+Y/2*3+0.02X/2=2.0*0.25
0.15*2+4Y=£Ø0.24-0.02£©*X
½āµĆ£ŗX=5 Y=0.2
æ¼µć£ŗĀĮŗĶĆ¾”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹŌŃłXÓÉŃõ»ÆŃĒĢśŗĶŃõ»ÆĶ×é³É£¬Č”ÖŹĮæĻąµČµÄĮ½·ŻŹŌŃł°“ĻĀĶ¼½ųŠŠŹµŃé£ŗ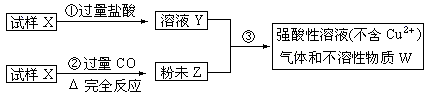
£Ø1£©ĒėŠ“³ö²½¾Ū¢ŪÖŠ·¢ÉśµÄČ«²æ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ____________________________________”£
£Ø2£©ŅŖŹ¹ŹŌŃłX×Ŗ±äĪŖ·ŪÄ©Z”£³żĮĖCOĶā£¬»¹æÉŅŌŹ¹ÓĆ ”£
| A£®ĒāĘų | B£®½¹Ģæ | C£®ĀČĘų | D£®ŃõĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĮŠĪļÖŹÖ®¼äÓŠČēĻĀ·“Ó¦¹ŲĻµ£ŗ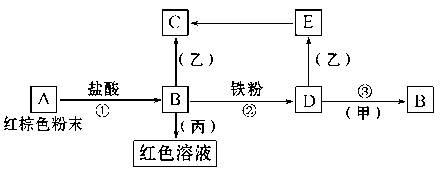
ŅŃÖŖÓÉE×Ŗ»Æ³ÉCµÄĻÖĻóŹĒ£ŗ°×É«³ĮµķŃøĖŁ±äĪŖ»ŅĀĢÉ«,×īŗó±äĪŖŗģŗÖÉ«”£»Ų“š£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗ¼×________,±ū________”£
£Ø2£©Š“³öE”śC·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ___________________________”£ĪŖĮĖ»ńµĆE£¬æÉŅŌÓĆŠĀÅäÖʵÄDČÜŅŗÓėÓĆ²»ŗ¬O2µÄÕōĮóĖ®ÅäÖʵÄŅŅČÜŅŗ·“Ó¦Öʱø”£
£Ø3£©ÓĆĻąÓ¦¾§ĢåÅäÖĘÉĻŹöDČÜŅŗŹ±Äć¾õµĆ»¹Šč¼ÓČė ”£
£Ø4£©Š“³öFeÓėH20ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)ĄūÓĆ»Æѧ·“Ó¦æÉŅŌÖʱøŠķ¶ąĪļÖŹ”£ŹµŃéŹŅÓĆĶÖʱøNO2µÄĄė×Ó·½³ĢŹ½ĪŖ_____________ ______”£
£Ø2£©¹¤ŅµÉĻ£¬»ĘĶæó£ØÖ÷ŅŖ³É·ÖCuFeS2£©ŹĒĢįČ”ĶµÄÖ÷ŅŖŌĮĻ£¬æɲÉÓĆ»š·ØČÜĮ¶¹¤ŅÕÉś²śĶ£¬øĆ¹¤ŅÕµÄÖŠ¼ä¹ż³Ģ»į·¢Éś·“Ó¦£ŗCu2S£«2Cu2O===6Cu+SO2”ü”£øĆ·“Ó¦ÖŠ»¹Ō¼ĮĪŖ_______ (Ģī»ÆѧŹ½)£¬ĆæÉś³É1mol Cu£¬·“Ó¦ÖŠ×ŖŅʵē×ÓµÄĪļÖŹµÄĮæĪŖ___________”£
»ĘĶæóŅ±Į¶Ķ²śÉśµÄĀÆŌü£Øŗ¬Fe2O3”¢FeO”¢SiO2”¢Al2O3£©æÉÖʱøFe2O3”£·½·ØĪŖ£ŗ
¢ŁÓĆĻ”ŃĪĖį½žČ”ĀÆŌü£¬¹żĀĖ”£ ¢ŚĀĖŅŗĻČŃõ»Æ£¬ŌŁ¼ÓČė¹żĮæNaOHČÜŅŗ£¬¹żĀĖ£¬½«³ĮµķĻ“µÓ”¢øÉŌļ”¢ģŃÉÕµĆFe2O3”£ ¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
a£®ĶعżÉĻŹö¢Ś£¬ĀÆŌüÖŠµÄAl2O3±ä³ÉĮĖ £ØŠ“Ąė×Ó£©”£
b£®Ń”ÓĆĢį¹©µÄŹŌ¼Į£¬Éč¼ĘŹµŃéŃéÖ¤ĀÆŌüÖŠŗ¬ÓŠFeO”£
Ģį¹©µÄŹŌ¼Į£ŗĻ”ŃĪĖį Ļ”ĮņĖį KSCNČÜŅŗ ĖįŠŌKMnO4ČÜŅŗ NaOHČÜŅŗ µāĖ®
ĖłŃ”ŹŌ¼ĮĪŖ ”£
Ö¤Ć÷ĀÆŌüÖŠŗ¬ÓŠFeOµÄŹµŃéĻÖĻóĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ĶŹĒČĖĄą×īŌēŹ¹ÓĆµÄ½šŹōÖ®Ņ»£¬ŅŌĻĀŹĒŅ±Į¶ĶµÄŅ»øö·“Ó¦£ŗ
Cu2S+2Cu2O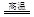 6Cu+SO2
6Cu+SO2
øĆ·“Ó¦ÖŠ±»Ńõ»ÆµÄŌŖĖŲŹĒ £¬Čō·“Ó¦ÖŠ×ŖŅĘ1molµē×Ó£¬µĆµ½Cu mol”£
£Ø2£©ĻĀĮŠĮ½ÖÖ·½·Ø¾łæÉŅŌÖʱøCuSO4”£
·½·ØŅ»£ŗ2Cu+O2 2CuO£¬CuO+H2SO4=CuSO4+H2O
2CuO£¬CuO+H2SO4=CuSO4+H2O
·½·Ø¶ž£ŗCu+2H2SO4£ØÅØ£© CuSO4+SO2”ü+2H2O
CuSO4+SO2ӟ+2H2O
¢Ł¼ŁČēij¹¤³§ÓūÉś²śCuSO4£¬ĒėŃ”ŌńŅ»ÖÖ·½·Ø£¬²¢ĖµĆ÷ĄķÓÉ£ŗ
ӣ
¢ŚÓŠĶ¬Ń§ČĻĪŖ£¬Éś³ÉµČĮæµÄĮņĖįĶĮ½ÖÖ·½·ØĻūŗĵÄÄÜĮæĻąĶ¬£¬ŅņĪŖ·“Ó¦¶¼ŹĒĶ×Ŗ±äĪŖCuSO4£¬ÄćČĻĪŖÉĻŹöĖµ·Ø £ØĢī”°ÕżČ·”±»ņ”°²»ÕżČ·”±£©£¬ŌŅņŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øł¾ŻĻĀĮŠ×Ŗ»Æ¹ŲĻµŅŌ¼°ĻÖĻó»Ų“š£ŗ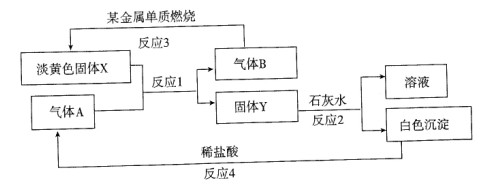
£Ø1£©¹ĢĢåXµÄĆū³ĘŹĒ £¬YµÄ»ÆѧŹ½ŹĒ ”£
£Ø2£©Š“³ö·“Ó¦lµÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©Š“³ö·“Ó¦2µÄ»Æѧ·½³ĢŹ½ ”£
£Ø4£©Čō15£®6g XŗĶ×ćĮæĖ®·“Ó¦£¬×ŖŅĘ mol e-”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓÉ»ĘĶæó(Ö÷ŅŖ³É·ÖŹĒCuFeS2)Į¶ÖĘ¾«ĶµÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ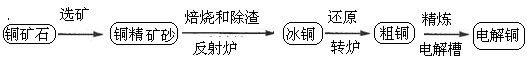
£Ø1£©ŌŚ·“ÉäĀÆÖŠ£¬°ŃĶ¾«æóÉ°ŗĶŹÆӢɰ»ģŗĻ¼ÓČȵ½1000”ę×óÓŅ£¬»ĘĶæóÓėæÕĘų·“Ӧɜ³ÉCuŗĶFeµÄµĶ¼ŪĮņ»ÆĪļ£¬ĒŅ²æ·ÖFeµÄĮņ»ÆĪļ×Ŗ»ÆĪŖµĶ¼ŪŃõ»ÆĪļ”£øĆ¹ż³ĢÖŠĮ½øöÖ÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”¢ £¬·“ÉäĀÆÄŚÉś³ÉĀÆŌüµÄÖ÷ŅŖ³É·ÖŹĒ £»
£Ø2£©±łĶ(Cu2SŗĶFeS»„ĻąČŪŗĻ¶ų³É)ŗ¬CuĮæĪŖ20%”«50%”£×ŖĀÆÖŠ£¬½«±łĶ¼ÓČŪ¼Į(ŹÆӢɰ)ŌŚ1200”ę×óÓŅ“µČėæÕĘų½ųŠŠ“µĮ¶”£±łĶÖŠµÄCu2S±»Ńõ»Æ³ÉCu2O£¬Éś³ÉµÄCu2OÓėCu2S·“Ó¦£¬Éś³Éŗ¬CuĮæŌ¼ĪŖ98.5%µÄ“ÖĶ£¬øĆ¹ż³Ģ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”¢ £»
£Ø3£©“ÖĶµÄµē½ā¾«Į¶ČēĶ¼ĖłŹ¾”£ŌŚ“ÖĶµÄµē½ā¹ż³ĢÖŠ£¬“ÖĶ°åŹĒĶ¼ÖŠµē¼« (ĢīĶ¼ÖŠµÄ×ÖÄø)£»ŌŚµē¼«dÉĻ·¢ÉśµÄµē¼«·“Ó¦Ź½ĪŖ £»Čō“ÖĶÖŠ»¹ŗ¬ÓŠAu”¢Ag”¢Fe£¬ĖüĆĒŌŚµē½ā²ŪÖŠµÄ“ęŌŚŠĪŹ½ŗĶĪ»ÖĆĪŖ ”£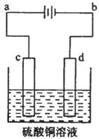
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½šŹōĀĮÖŹĒįĒŅÓŠĮ¼ŗƵķĄøÆŹ“ŠŌ£¬ŌŚ¹ś·Ą¹¤ŅµÖŠÓŠ·Ē³£ÖŲŅŖµÄ×÷ÓĆ£¬ĄūÓĆĀĮČČ·“Ó¦Ķź³ÉøÖ¹ģµÄŗø½Ó·Ē³£·½±ćŃøĖŁ”£Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©ŌŚĢŲÖĘĀ©¶·ÖŠ½«ĀĮČČ¼Į»ģŗĻ¾łŌČŗó£¬Ņż·¢ĀĮČČ·“Ó¦µÄ²Ł×÷ŹĒ£ŗ £»
£Ø2£©¹čÓėĀĮĶ¬ÖÜĘŚ”£µŲæĒĄļ¹č”¢ĀĮµÄŗ¬Įæ¹č ĀĮ£ØĢī£¾,£¼»ņ=£©”£SiO2ŹĒ¹čĖįŃĪ²£Į§£ØNa2CaSi6O14£©Ö÷ŅŖ³É·Ö£¬Na2CaSi6O14Ņ²æÉŠ“³ÉNa2O”¤CaO”¤6SiO2”£ÄĘ³¤ŹÆ£ØNaAlSi3O8£©µÄŃõ»ÆĪļŠĪŹ½ £¬³¤ŹÆŹĒĀĮ¹čĖįŃĪ£¬²»Ķ¬Ąą³¤ŹÆĘäŃõŌ×ÓµÄĪļÖŹµÄĮæ·ÖŹżĻąĶ¬”£ÓÉ“ĖæÉĶĘÖŖøĘ³¤ŹÆµÄ»ÆѧŹ½ĪŖ £»
£Ø3£©Ä³ĀĮŗĻ½šÓÉAl”¢Si”¢Cu”¢Mg×é³É”£¢Ł³ĘČ”100gøĆĀĮŗĻ½šŃłĘ·£¬·Ö³ÉµČÖŹĮæµÄA”¢BĮ½·Ż”£ĻņA·Ż¼ÓČė×ćĮæNaOHČÜŅŗ£¬B·Ż¼ÓČė×ćĮæµÄĻ”ŃĪĖį”£¢Ś“żĮ½·Ż·“Ó¦Īļ¶¼³ä·Ö·“Ó¦Ö®ŗó£¬³ĘµĆĀĖŌüÖŹĮæĻą²ī1.60g£¬ŹÕ¼ÆµĆµ½µÄĮ½·ŻĘųĢåµÄĢå»żĻą²ī2240mL(±ź×¼×“æöĻĀ)”£Ōņѳʷ֊SiŗĶMgµÄĪļÖŹµÄĮæ·Ö±šŹĒ ŗĶ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶žĮņ»ÆŃĒĢśŹĒLi/FeS2µē³ŲµÄÕż¼«»īŠŌĪļÖŹ£¬æÉÓĆĖ®ČČ·ØŗĻ³É”£FeSO4”¢Na2S2O3”¢S¼°H2OŌŚ200”ęĮ¬Šų·“Ó¦24Š”Ź±£¬ĖÄÖÖĪļÖŹŅŌµČĪļÖŹµÄĮæ·“Ó¦£¬ŌŁŅĄ“ĪÓĆCS2”¢H2OĻ“µÓ”¢øÉŌļ¼°¾§»ÆŗóµĆµ½”£
£Ø1£©ŗĻ³ÉFeS2Ąė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓĆĖ®Ļ“µÓŹ±£¬ČēŗĪÖ¤Ć÷S042-¼ŗ³ż¾””” ”£
£Ø3£©¼ŗÖŖ1.20gFeS2ŌŚO2ÖŠĶźČ«Č¼ÉÕÉś³ÉFe2O3ŗĶSO2ĘųĢå·Å³ö8.52kJČČĮ棬FeS2Č¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ”” ”£
£Ø4£©Č”ÉĻŹöÖʵƵÄÕż¼«²ÄĮĻ1.1200g (¼Ł¶ØÖ»ŗ¬FeSŅ»ÖÖŌÓÖŹ)£¬ŌŚ×ćĮæµÄŃõĘųĮ÷ÖŠ³ä·Ö¼ÓČČ£¬×īŗóµĆ0.8000gŗģ×ŲÉ«¹ĢĢ壬ŌņøĆÕż¼«²ÄĮĻÖŠFeS2µÄÖŹĮæ·ÖŹż(Š“³ö¼ĘĖć¹ż³Ģ)”£
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com