
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
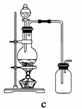
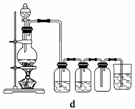
(1)��ʳ�γ���������K����Ca2����Mg2����Fe3����SO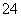 ���������ӣ�ʵ�����ᴿNaCl���������£�
���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ������Ȼ�̼
������ȥ��Һ���е�Ca2����Mg2����Fe3����SO ���ӣ�ѡ��a���������Լ������μ�˳������Ϊ______________________(ֻ�ѧʽ)��
���ӣ�ѡ��a���������Լ������μ�˳������Ϊ______________________(ֻ�ѧʽ)��
��ϴ�ӳ�ȥNaCl������渽��������KCl�����õ��Լ�Ϊ__________��
(2)���ᴿ��NaCl����500 mL 4.00 mol��L��1 NaCl��Һ������������ҩ�ס��������⣬����__________(����������)��
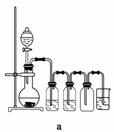
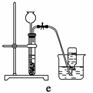
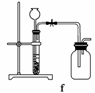
(4)ʵ�����Ʊ�H2��Cl2ͨ���������з�Ӧ��
Zn��H2SO4===ZnSO4��H2��
MnO2��4HCl(Ũ)MnCl2��Cl2����2H2O
�ݴˣ�����������������ѡ���Ʊ����ռ�H2��װ��________(�����)���Ʊ����ռ��������Cl2��װ��________(�����)��
��ѡ���Ʊ������װ�ã�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijԭ��ص����ӷ���ʽ��Zn��Cu2��===Zn2����Cu����ԭ��������ȷ����(����)
| ѡ�� | ���� | ���� | �������Һ |
| A | Zn | Al | CuSO4 |
| B | Zn | Cu | CuSO4 |
| C | Cu | Zn | CuCl2 |
| D | Cu | Zn | ZnCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͨ������£�A�ǹ��嵥�ʡ�������ͼ��ת����ϵ���ش��й����⣺
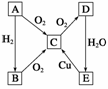
(1)д�����з���ʽ��
A��__________��C��__________��E��________��
(2)д��ָ����Ӧ�Ļ�ѧ����ʽ��
��B��C��________________________________________________________________________��
��C��D��________________________________________________________________________��
��E��C��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС����̽�������仯����������Ժͻ�ԭ�ԡ���ش��������⣺
(1)����ͷ�ι��⣬����Ϊ��ʵ��ز����ٵ�һ�ֲ���������______��
(2)����������������ʵ�鱨�棺
һ��ʵ��Ŀ�ģ�_______________________________________________________��
�����Լ������ۡ�FeCl3��Һ��FeCl2��Һ����ˮ��пƬ��ͭƬ��KSCN��Һ��
����ʵ���¼(��б�߲��ֲ�����д)��
| ��� | ʵ������ | ʵ������ | ���ӷ���ʽ | ʵ����� |
| �� | ��FeCl2��Һ�е���������ˮ | ��Һ��dz��ɫ��Ϊ�ػ�ɫ | Fe2�����л�ԭ�� | |
| �� | ��FeCl2��Һ�м���пƬ | Zn��Fe2��=== Zn2����Fe | ||
| �� | ��FeCl3��Һ�м����������� | Fe��2Fe3��===3Fe2�� | Fe3������������ | |
| �� | Fe3������������ |
�ġ�ʵ����ۣ�
Feֻ�л�ԭ�ԣ�Fe3��ֻ�������ԣ�Fe2�����������ԣ����л�ԭ�ԡ�
(3)�������Ͻ����жϣ����������м��������ԣ����л�ԭ�Ե���______��(�����)
A��Cl2 B��Na��C��Na��
D��Cl�� E��SO2��F��NO2
(4)����������Һ���ױ���������ʵ������Ҫ��������������Һ����ô������������ҺʱӦ����η�ֹ�����α�������_________________________________________________��
������Fe2���ļ��鷽����_________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���þ��������ȷ����(����)
A��þ��ʧ�������CO2��������
B��þ������ˮ��Ӧ�ų�H2
C��þ����������ʢ��Ũ����
D��þ�ڿ�����ȼ�ղ��ﲢ��ȫ��MgO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��25 �桢1.01��105 Pa�£���22 g CO2ͨ��1 mol��L-1 NaOH��Һ750 mL�г�ַ�Ӧ����÷�Ӧ�ų�x kJ�������ڸ������£�1 mol CO2 ͨ��2 mol��L-1 NaOH��Һ1 L�г�ַ�Ӧ�ų�y kJ��������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ����ʽ��( )
ͨ��2 mol��L-1 NaOH��Һ1 L�г�ַ�Ӧ�ų�y kJ��������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ����ʽ��( )
A��CO2(g)+NaOH(aq)====NaHCO3(aq) ��H=-(2y��x) kJ��mol��1
B��CO2(g)+NaOH(aq)====NaHCO3(aq) ��H=-(2x��y) kJ��mol��1
C��CO2(g)��NaOH(aq)====NaHCO3(aq) ��H=��(4x��y) kJ��mol��1
D��CO2(g)��NaOH(l)====NaHCO3(l) ��H=��(8x��2y) kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������¶���ڿ����У�����������ԭ��Ӧ�����ʵ���
A.�������� B.�Ȼ����� C.ʳ�� D.̼������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ʵ�������������һ���� �� ��
A��ȡ5mL 0.1 mol/L KI��Һ���μ�0.1mol/LFeCl3��Һ5��6�Σ���������2 mLCCl4�������ȡ�ϲ���Һ���μ�KSCN��Һ����Һ����Ѫ��ɫ��֤����Һ�л�����Fe3+
B�������������ⸯʴʵ���У��ڸ�����Χ����K3[Fe(CN)6]��Һ�������ɫ����
C��ȼ�ϵ��ʵ���У���KNO3��Һ��Na2SO4��Һ��������ˮ��Ч��Ҫ�õö�
D�����ǵġ��������ʵ����ֻ������Ũ�������ˮ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com