
��1��������ʯ�����ڳ������Ƭ�������²�����ϩ�����������ʵ�ʵ�飬����������⡣
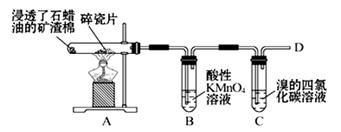
��A�����Ƭ�������� ��
��B�з�Ӧ������ ��C��ʵ�������� ��
��2����ȡ�����飨CH3CH2Cl������ѷ����ǣ��û�ѧ����ʽ��ʾ����
��
��3���ÿ�������ˮ��SO2 ��Һ�еĻ�ѧ����ʽ��
 ��
��
��4���Ѹɺ������� �����������ƣ������ա����պ�ĺ�������ˮ�У����˺����Һ���ữ��ӹ�����������ӷ���ʽ
��5�����Թ�A�м���3 mL �Ҵ���Ȼ������Թܱ���������2 mL Ũ�����2 mL ���ᣬ����ͼ��ʾ���Ӻ�װ����ȡ����������
���Թ�B��ʢ�ű���Na2CO3��Һ�������ǣ��к����ᡢ�ܽ��Ҵ��� ��
��ʵ����ɺ���Ҫ����B�е�Һ�������Ҫ�õ��IJ���������Ҫ�� ��
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����һ��ѧ�������¡��л������3.2.1��ϩ(�˽̰����2) ���ͣ�058
������ʯ�����ڳ������Ƭ�������²���C2H4������C2H4���ʵ�ʵ�飬������и����⣮
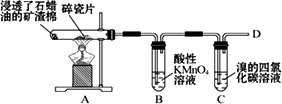
(1)B����Һ��ɫ������Ϊ��ϩ��________��
(2)C�з�����Ӧ�Ļ�ѧ����ʽ________��
(3)��D����ȼʱ������еIJ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��12�֣�
��1��������ʯ�����ڳ������Ƭ�������²�����ϩ�����������ʵ�ʵ�飬����������⡣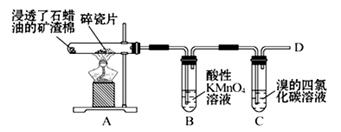
��A�����Ƭ�������� ��
��B�з�Ӧ������ ��C��ʵ�������� ����2����ȡ�����飨CH3CH2Cl������ѷ����ǣ��û�ѧ����ʽ��ʾ���� ����3���ÿ�������ˮ��SO2��Һ�еĻ�ѧ����ʽ�� ����4���Ѹɺ������� �����������ƣ������ա����պ�ĺ�������ˮ�У����˺����Һ���ữ��ӹ�����������ӷ���ʽ
��5�����Թ�A�м���3 mL �Ҵ���Ȼ������Թܱ���������2 mL Ũ�����2 mL ���ᣬ����ͼ��ʾ���Ӻ�װ����ȡ����������
���Թ�B��ʢ�ű���Na2CO3��Һ�������ǣ��к����ᡢ�ܽ��Ҵ��� ��
��ʵ����ɺ���Ҫ����B�е�Һ�������Ҫ�õ��IJ���������Ҫ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ��ԭ�и�һ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��1��������ʯ�����ڳ������Ƭ�������²�����ϩ�����������ʵ�ʵ�飬����������⡣
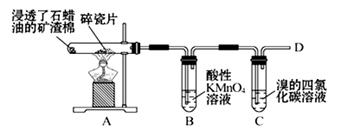
��A�����Ƭ�������� ��
��B�з�Ӧ������ ��C��ʵ�������� ��
����D����ȼǰ������еIJ����� ��
��2����ȡ�����飨CH3CH2Cl������ѷ����ǣ��û�ѧ����ʽ��ʾ���� ��
��3������ʯ�͵���һ��Ʒ�DZ�����д���ɱ����������Ļ�ѧ����ʽ�� ��
��4����ʵ����D����ϩȼ�յĻ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�
��1��������ʯ�����ڳ������Ƭ�������²�����ϩ�����������ʵ�ʵ�飬����������⡣
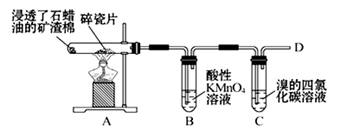
��A�����Ƭ�������� ��
��B�з�Ӧ������ ��C��ʵ�������� ����2����ȡ�����飨CH3CH2Cl������ѷ����ǣ��û�ѧ����ʽ��ʾ���� ����3���ÿ�������ˮ��SO2��Һ�еĻ�ѧ����ʽ�� ����4���Ѹɺ������� �����������ƣ������ա����պ�ĺ�������ˮ�У����˺����Һ���ữ��ӹ�����������ӷ���ʽ
��5�����Թ�A�м���3 mL �Ҵ���Ȼ������Թܱ���������2 mL Ũ�����2 mL ���ᣬ����ͼ��ʾ���Ӻ�װ����ȡ����������
���Թ�B��ʢ�ű���Na2CO3��Һ�������ǣ��к����ᡢ�ܽ��Ҵ��� ��
��ʵ����ɺ���Ҫ����B�е�Һ�������Ҫ�õ��IJ���������Ҫ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com