
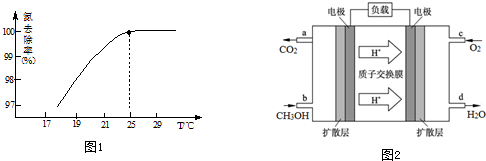
·ÖĪö £Ø1£©¢ŁŅŃÖŖ£ŗ¢ń£®N2£Øg£©+2H2£Øg£©ØTN2H4£Øl£©”÷H=+50.6kJ•mol-1
¢ņ.2H2£Øg£©+O2£Øg£©ØT2H2O£Øl£©”÷H=-571.6kJ•mol-1
øł¾ŻøĒĖ¹¶ØĀÉ£¬¢ņ-¢ńæÉµĆ£ŗN2H4£Øl£©+O2£Øg£©ØTN2£Øg£©+2H2O£Øl£©£»
¢ŚÓĆ“ĪĀČĖįÄĘŃõ»Æ°±£¬æÉŅŌµĆµ½N2H4µÄĻ”ČÜŅŗ£¬“ĪĀČĖįÄʱ»»¹ŌÉś³ÉNaCl£¬»¹ÓŠĖ®Éś³É£»
£Ø2£©¢ŁÉżøßĪĀ¶ČNH3µÄČܽā¶Č½µµĶ£»
¢ŚŃõ»ÆĆ¾ÄŃČÜÓŚĖ®ÖŠ£¬²»»įŠĪ³É¶ž“ĪĪŪČ¾£»
£Ø3£©¢Łøł¾Żµē×Ó×ŖŅĘŹŲŗć¼ĘĖćĻūŗÄŃõĘų£»
¢Ś¼×“¼ŗĶNO3-·“Ó¦×Ŗ»ÆĪŖĮ½ÖÖĪŽ¶¾ĘųĢ壬ӦŹĒÉś³ÉN2”¢CO2£¬·“Ó¦»¹Éś³ÉĖ®£»
£Ø4£©Ōµē³Ųøŗ¼«·¢ÉśŃõ»Æ·“Ó¦£¬ÓÉŹ¾ŅāĶ¼æÉÖŖ£¬¼×“¼ŌŚøŗ¼«Ź§Č„µē×ÓÉś³ÉCO2ÓėH+£®
½ā“š ½ā£ŗ£Ø1£©¢ŁŅŃÖŖ£ŗ¢ń£®N2£Øg£©+2H2£Øg£©ØTN2H4£Øl£©”÷H=+50.6kJ•mol-1
¢ņ.2H2£Øg£©+O2£Øg£©ØT2H2O£Øl£©”÷H=-571.6kJ•mol-1
øł¾ŻøĒĖ¹¶ØĀÉ£¬¢ņ-¢ńæÉµĆ£ŗN2H4£Øl£©+O2£Øg£©ØTN2£Øg£©+2H2O£Øl£©£¬Ōņ”÷H=-571.6kJ•mol-1
-50.6kJ•mol-1=-622.2kJ•mol-1£¬
¹Ź“š°øĪŖ£ŗ-622.2£»
¢ŚÓĆ“ĪĀČĖįÄĘŃõ»Æ°±£¬æÉŅŌµĆµ½N2H4µÄĻ”ČÜŅŗ£¬“ĪĀČĖįÄʱ»»¹ŌÉś³ÉNaCl£¬»¹ÓŠĖ®Éś³É£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ2NH3+NaClO=N2H4+NaCl+H2O£¬
¹Ź“š°øĪŖ£ŗ2NH3+NaClO=N2H4+NaCl+H2O£»
£Ø2£©¢ŁŌŚ25”ęĒ°£¬ÉżøßĪĀ¶ČµŖČ„³żĀŹŌö“óµÄŌŅņŹĒ£ŗŅņĪŖ°±ĘųČܽā¶ČĖęĪĀ¶ČÉżøߣ¬Čܽā¶Č¼õŠ”£¬ÓŠĄūÓŚNH3µÄŅŻ³ö£¬
¹Ź“š°øĪŖ£ŗÉżøßĪĀ¶ČNH3µÄČܽā¶Č½µµĶ£¬ÓŠĄūÓŚNH3µÄŅŻ³ö£»
¢ŚŹ£ÓąµÄŃõ»ÆĆ¾£¬ŹĒÄŃČÜÓŚĖ®µÄĪļÖŹ£¬ŅŌ³ĮµķµÄŠĪŹ½Åųö£¬²»»įŠĪ³É¶ž“ĪĪŪČ¾£¬
¹Ź“š°øĪŖ£ŗŃõ»ÆĆ¾ÄŃČÜÓŚĖ®ÖŠ£¬ŅŌ³ĮµķµÄŠĪŹ½Åųö£¬Ņņ“Ė²»»įŠĪ³É¶ž“ĪĪŪČ¾£»
£Ø3£©¢Ł14gļ§Ģ¬µŖŌŖĖŲĪļÖŹµÄĮæĪŖ$\frac{14g}{14g/mol}$=1mol£¬øł¾Żµē×Ó×ŖŅĘŹŲŗć£¬×Ŗ»ÆĪŖĻõĢ¬µŖŌŖĖŲŹ±ŠčŃõµÄĪļÖŹµÄĮæĪŖ$\frac{1mol”Į[5-£Ø-3£©]}{4}$=2mol£¬ŠčŅŖŃõĘųÖŹĮæĪŖ2mol”Į32g/mol=64g£¬
¹Ź“š°øĪŖ£ŗ64g£»
¢Ś¼×“¼ŗĶNO3-·“Ó¦×Ŗ»ÆĪŖĮ½ÖÖĪŽ¶¾ĘųĢ壬ӦŹĒÉś³ÉN2”¢CO2£¬·“Ó¦»¹Éś³ÉĖ®£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗ6NO3-+5CH3OH+6H+=3N2”ü+5CO2”ü+13H2O£¬
¹Ź“š°øĪŖ£ŗ6NO3-+5CH3OH+6H+=3N2”ü+5CO2”ü+13H2O£»
£Ø4£©Ōµē³Ųøŗ¼«·¢ÉśŃõ»Æ·“Ó¦£¬ÓÉŹ¾ŅāĶ¼æÉÖŖ£¬¼×“¼ŌŚøŗ¼«Ź§Č„µē×ÓÉś³ÉCO2ÓėH+£¬øŗ¼«µē¼«·“Ó¦Ź½ĪŖ£ŗCH3OH+H2O-6e-=CO2”ü+6H+£¬
¹Ź“š°øĪŖ£ŗCH3OH+H2O-6e-=CO2”ü+6H+£®
µćĘĄ ±¾Ģā±Č½Ļ×ŪŗĻ£¬Éę¼°·“Ó¦ČČ¼ĘĖć”¢µē¼«·“Ó¦Ź½ŹéŠ“”¢Ńõ»Æ»¹Ō·“Ó¦¼ĘĖćµČ£¬ŹĒ¶Ōѧɜ×ŪŗĻÄÜĮ¦æ¼²é£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ¾Ę¾« | B£® |  Ļ”H2SO4 | C£® |  ¾Ę¾« | D£® |  Ļ”H2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪĀ¶Č | B£® | Ń¹Ēæ | C£® | ÅØ¶Č | D£® | “߻ƼĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ļ”ĻõĖįÖŠ¼ÓČė¹żĮæĢś·Ū£ŗFe+4H++NO${\;}_{3}^{-}$ØTFe3++NO”ü+2H2O | |
| B£® | ĻņNH4HSO4ČÜŅŗÖŠÖšµĪµĪČėBa£ØOH£©2ČÜŅŗÖĮĒ”ŗĆ³ĮµķĶźČ«2OH-+Ba2++2H++SO${\;}_{4}^{2-}$ØT2H2O+BaSO4”ż | |
| C£® | ĻņŃĒĮņĖį±µ¹ĢĢåÖŠ¼ÓČėĻ”ĻõĖį£ŗ3BaSO3+2H++2NO${\;}_{3}^{-}$ØT3BaSO4”ż+2NO”ü+H2O | |
| D£® | ĻņCa£ØClO£©2ČÜŅŗÖŠĶØČė×ćĮæCO2£ŗCa2++CO2+H2OØTCaCO3”ż+2H+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹÆ»ŅŹÆ | B£® | Ca£ØOH£©2 | C£® | CaCO3 | D£® | CH4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
 ĻĀĮŠø÷×éĪļÖŹÖŠ£¬ĪļÖŹÖ®¼äĶعżŅ»²½·“Ó¦¾ĶÄÜŹµĻÖČēĶ¼ĖłŹ¾×Ŗ»ÆµÄŹĒ£Ø””””£©
ĻĀĮŠø÷×éĪļÖŹÖŠ£¬ĪļÖŹÖ®¼äĶعżŅ»²½·“Ó¦¾ĶÄÜŹµĻÖČēĶ¼ĖłŹ¾×Ŗ»ÆµÄŹĒ£Ø””””£©| ±ąŗÅ | a | b | c |
| A | Na | Na2O | NaOH |
| B | Al2O3 | NaAlO2 | Al£ØOH£©3 |
| C | CO2 | Na2CO3 | NaHCO3 |
| D | Fe | FeCl3 | Fe£ØOH£©3 |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com