
| A£® | Ņ»¶Ø²»“ęŌŚµÄĄė×ÓŹĒMg2+”¢Ba2+£¬²»ÄÜČ·¶ØŹĒ·ńŗ¬ÓŠK+”¢NO3- | |
| B£® | Ņ»¶Ø“ęŌŚµÄĄė×ÓŹĒCO32-”¢SO42-£¬CO32-µÄĪļÖŹµÄĮæÅضČĪŖ0.25mol•L-1£¬SO42-µÄĪļÖŹµÄĮæÅضČĪŖ0.1mol•L-1 | |
| C£® | Ņ»¶Ø“ęŌŚµÄĄė×ÓŹĒCO32-”¢SO42-”¢K+£¬ĘäÖŠK+ÅØ¶Č”Ż0.2mol•L-1 | |
| D£® | Ņ»¶Ø“ęŌŚµÄĄė×ÓŹĒCO32-”¢SO42-”¢K+£¬ĘäÖŠK+ÅضČĪŖ0.2mol•L-1 |
·ÖĪö I£®ĻņøĆČÜŅŗÖŠ¼ÓČė×ćĮæĻ”ŃĪĖį£¬ŌŚ±ź×¼×“æöĻĀ·Å³ö0.56LĘųĢå£Ø²»æ¼ĀĒĘųĢåČܽā£©£¬ĖµĆ÷Ņ»¶Øŗ¬CO32-£¬ŌņŅ»¶Ø²»ŗ¬Mg2+”¢Ba2+£¬Éś³ÉµÄĘųĢåĪŖ¶žŃõ»ÆĢ¼ĘųĢ壬ĪļÖŹµÄĮæn£ØCO2£©=n£ØCO32-£©=$\frac{0.56L}{22.4L/mol}$=0.025mol£»
II£®ĻņIÖŠĖłµĆµÄĀĖŅŗÖŠµĪ¼Ó×ćĮæBaCl2ČÜŅŗ²śÉś°×É«³Įµķ2.33g£¬ĖµĆ÷ČÜŅŗÖŠŅ»¶Øŗ¬SO42-£¬°×É«³ĮµķĪŖĮņĖį±µ£¬ĪļÖŹµÄĮæn£ØBaSO4£©=n£ØSO42-£©=$\frac{2.33g}{233g/mol}$=0.01mol£»
ČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓĪŖCO32-”¢SO42-”¢Na+£¬Ņ»¶Ø²»“ęŌŚMg2+”¢Ba2+£¬K+”¢NO3-æÉŅŌøł¾ŻČÜŅŗÖŠµēŗÉŹŲŗć¼ĘĖć·ÖĪö£¬ČÜŅŗÖŠc£ØNa+£©=0.5mol/L£¬c£ØCO32-£©=$\frac{0.025mol}{0.1L}$=0.25mol/L£¬c£ØSO42-£©=$\frac{0.01mol}{0.1L}$=0.1mol/L£¬ŅõĄė×ÓĖł“ųøŗµēŗɶą£¬ŌņÅŠ¶ĻČÜŅŗÖŠŅ»¶Øŗ¬K+£¬ČōĻõĖįøłĄė×Ó²»“ęŌŚ£¬ČÜŅŗÖŠµēŗÉŹŲŗćĪŖ£¬c£ØNa+£©+c£ØK+£©=2c£ØCO32-£©+2c£ØSO42-£©£¬c£ØK+£©=0.2mol/L£¬Čō“ęŌŚĻõĖįøłĄė×Ó£¬Ōņc£ØK+£©£¾0.2mol/L£¬¾Ż“Ė·ÖĪöŃ”ĻīÅŠ¶Ļ£®
½ā“š ½ā£ŗI£®ĻņøĆČÜŅŗÖŠ¼ÓČė×ćĮæĻ”ŃĪĖį£¬ŌŚ±ź×¼×“æöĻĀ·Å³ö0.56LĘųĢå£Ø²»æ¼ĀĒĘųĢåČܽā£©£¬ĖµĆ÷Ņ»¶Øŗ¬CO32-£¬ŌņŅ»¶Ø²»ŗ¬Mg2+”¢Ba2+£¬Éś³ÉµÄĘųĢåĪŖ¶žŃõ»ÆĢ¼ĘųĢ壬ĪļÖŹµÄĮæn£ØCO2£©=n£ØCO32-£©=$\frac{0.56L}{22.4L/mol}$=0.025mol£»
II£®ĻņIÖŠĖłµĆµÄĀĖŅŗÖŠµĪ¼Ó×ćĮæBaCl2ČÜŅŗ²śÉś°×É«³Įµķ2.33g£¬ĖµĆ÷ČÜŅŗÖŠŅ»¶Øŗ¬SO42-£¬°×É«³ĮµķĪŖĮņĖį±µ£¬ĪļÖŹµÄĮæn£ØBaSO4£©=n£ØSO42-£©=$\frac{2.33g}{233g/mol}$=0.01mol£»
ČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓĪŖCO32-”¢SO42-”¢Na+£¬Ņ»¶Ø²»“ęŌŚMg2+”¢Ba2+£¬K+”¢NO3-æÉŅŌøł¾ŻČÜŅŗÖŠµēŗÉŹŲŗć¼ĘĖć·ÖĪö£¬ČÜŅŗÖŠc£ØNa+£©=0.5mol/L£¬c£ØCO32-£©=$\frac{0.025mol}{0.1L}$=0.25mol/L£¬c£ØSO42-£©=$\frac{0.01mol}{0.1L}$=0.1mol/L£¬ŅõĄė×ÓĖł“ųøŗµēŗɶą£¬ŌņÅŠ¶ĻČÜŅŗÖŠŅ»¶Øŗ¬K+£¬ČōĻõĖįøłĄė×Ó²»“ęŌŚ£¬ČÜŅŗÖŠµēŗÉŹŲŗćĪŖ£¬c£ØNa+£©+c£ØK+£©=2c£ØCO32-£©+2c£ØSO42-£©£¬c£ØK+£©=0.2mol/L£¬Čō“ęŌŚĻõĖįøłĄė×Ó£¬Ōņc£ØK+£©£¾0.2mol/L£¬
A£®·ÖĪöæÉÖŖ£¬K+Ņ»¶Ø“ęŌŚ£¬¹ŹA“ķĪó£»
B£®Ņ»¶Ø“ęŌŚµÄĄė×ÓŹĒCO32-”¢SO42-”¢K+£¬¹ŹB“ķĪó£»
C£®ÉĻŹö·ÖĪöæÉÖŖŅ»¶Ø“ęŌŚµÄĄė×ÓŹĒCO32-”¢SO42-”¢K+£¬ĘäÖŠK+ÅØ¶Č”Ż0.2mol•L-1£¬¹ŹCÕżČ·£»
D£®ČōĻõĖįøłĄė×Ó²»“ęŌŚ£¬ČÜŅŗÖŠµēŗÉŹŲŗćĪŖ£¬c£ØNa+£©+c£ØK+£©=2c£ØCO32-£©+2c£ØSO42-£©£¬c£ØK+£©=0.2mol/L£¬Čō“ęŌŚĻõĖįøłĄė×Ó£¬Ōņc£ØK+£©£¾0.2mol/L£¬¹ŹD“ķĪó£»
¹ŹŃ”C£®
µćĘĄ ±¾Ģāæ¼²éĄė×ÓŠŌÖŹ”¢·“Ó¦ĻÖĻóŗĶĄė×Ó·“Ó¦ŗóµÄ¶ØĮæ¹ŲĻµ£¬ÄѶČÖŠµČ£¬×¢ŅāÕĘĪÕĄė×Ó·“Ó¦£¬øł¾ŻµēŗÉŹŲŗćÅŠ¶ĻK+ŹĒ·ń“ęŌŚ£¬ŹĒ±¾ĢāµÄÄŃµć”¢Ņדķµć£¬ÄѶČÖŠµČ£®


 Ķ¬²½ĒįĖÉĮ·Ļ°ĻµĮŠ“š°ø
Ķ¬²½ĒįĖÉĮ·Ļ°ĻµĮŠ“š°ø æĪæĪĶØæĪ³Ģ±ź×¼Ė¼Ī¬·½·ØÓėÄÜĮ¦ŃµĮ·ĻµĮŠ“š°ø
æĪæĪĶØæĪ³Ģ±ź×¼Ė¼Ī¬·½·ØÓėÄÜĮ¦ŃµĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
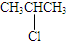 ¢Ū
¢Ū ¢Ü
¢Ü £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | N2 | B£® | NO2 | C£® | NO | D£® | N2O3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĖįŅŅõ„µÄ»ÆѧŹ½ĪŖ£ŗC4H8O2 | B£® | ±½µÄ½į¹¹¼ņŹ½ĪŖ£ŗ | ||
| C£® | ĖÄĀČ»ÆĢ¼µÄµē×ÓŹ½ĪŖ£ŗ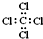 | D£® | »·±ūĶéµÄ¼üĻߏ½ĪŖ£ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | AlCl3ČÜŅŗÓėÉÕ¼īČÜŅŗ·“Ó¦£¬µ±n£ØOH-£©£ŗn£ØAl3+£©=7£ŗ2Ź±£¬2Al3++7OH-ØTAl£ØOH£©3”ż+AlO2-+2H2O | |
| B£® | CuCl2ČÜŅŗÓėNaHSČÜŅŗ·“Ó¦£¬µ±n£ØCuCl2£©£ŗn£ØNaHS£©=1£ŗ2Ź±Cu2++2HS-ØTCuS”ż+H2S”ü | |
| C£® | Cl2ÓėFeBr2ČÜŅŗ·“Ó¦£¬µ±n£ØCl2£©£ŗn£ØFeBr2£©=1£ŗ1Ź±£¬2Fe2++4Br-+3Cl2ØT2 Fe3++2Br2+6Cl- | |
| D£® | FeÓėĻ”ĻõĖį·“Ó¦£¬µ±n£ØFe£©£ŗn£ØHNO3£©=3£ŗ8Ź±£¬3Fe+2NO3-+8H+ØT3Fe2++2NO”ü+4H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | “×Ėįļ§ | B£® | ĮņĖį±µ | C£® | ĮņĒč»ÆĢś | D£® | µā»ÆĒā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĶŠÅĢĢģĘ½³ĘČ”3.25g NaCl | |
| B£® | ÓĆĖįŹ½µĪ¶Ø¹ÜĮæČ”20.00mL KMnO4ČÜŅŗ | |
| C£® | ÓĆĮæĶ²ĮæČ”10.51 mLŃĪĖį | |
| D£® | ÓĆČŻĮæĘæÅäÖĘ216mL 0.1mol/L NaOHČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅ“¼ÓėÅØĒāäåĖį·“Ó¦ CH3CH2OH+HBr$\stackrel{”÷}{”ś}$CH3CH2Br+H2O | |
| B£® | äåŅŅĶéÓėĒāŃõ»ÆÄĘČÜŅŗ¹²ČČ CH3CH2Br+NaOH$”ś_{”÷}^{Ė®}$CH3CH2OH+NaBr | |
| C£® | ±½·ÓÄĘÖŠĶØČėÉŁĮæµÄ¶žŃõ»ÆĢ¼ 2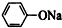 +CO2+H2O”ś2 +CO2+H2O”ś2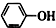 +Na2CO3 +Na2CO3 | |
| D£® | ŅŅČ©“ß»ÆŃõ»Æ 2CH3CHO+O2$”ś_{”÷}^{“߻ƼĮ}$2CH3COOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ²Ł×÷ | ĻÖĻó | ½įĀŪ | |
| A | ¢ŁµĪ¼ÓĻ”HNO3 ¢ŚµĪ¼ÓBaCl2ČÜŅŗ | ĪŽĆ÷ĻŌĻÖĻó °×É«³Įµķ | ŌČÜŅŗŅ»¶Øŗ¬Ag+ |
| B | µĪ¼ÓĻ”ŃĪĖį | ÓŠ“óĮæĘųÅŻ²śÉś | ŌČÜŅŗŅ»¶Øŗ¬CO32- |
| C | ¢ŁµĪ¼ÓĻ”HCl ¢ŚµĪ¼ÓAgNO3ČÜŅŗ | ĪŽĆ÷ĻŌĻÖĻó °×É«³Įµķ | ŌČÜŅŗŅ»¶Øŗ¬Cl- |
| D | ¢Ł¼ÓKSCNČÜŅŗ ¢ŚµĪ¼ÓĀČĖ® | ĪŽĆ÷ĻŌĻÖĻó ČÜŅŗ³ŹŗģÉ« | ŌČÜŅŗŅ»¶Øŗ¬Fe2+ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com