
Ķł200mL NaOHČÜŅŗÖŠĶØČėCO2£¬³ä·Ö·“Ó¦ŗó£¬ŌŚ¼õŃ¹ŗĶµĶĪĀĻĀ£¬Š”ŠÄ½«ČÜŅŗÕōøÉ£¬µĆ°×É«¹ĢĢåM”£ĶØČėCO2µÄĢå»żV£ØCO2£©£ØmL£©£Ø±ź×¼×“æöĻĀ£©ÓėMµÄÖŹĮæ£Øg£©µÄ¹ŲĻµČēĻĀĶ¼”£ŹŌĶعż·ÖĪöŗĶ¼ĘĖć»Ų“šĻĀĮŠĪŹĢā£ØŅŖĒ󊓳ö¼ņŅŖ¼ĘĖć¹ż³Ģ£©£ŗ
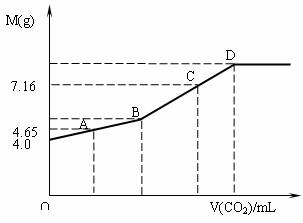
£Ø1£©BµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½_________£¬ĶØČėCO2µÄĢå»żĪŖ______________mL”£
£Ø2£©DµćŹ±£¬ĶØČėCO2µÄĢå»żĪŖ__________mL”£
£Ø3£©CµćŹ±£¬ĶØČėCO2µÄĢå»żĪŖ__________mL”£
£Ø4£©Č”×é³ÉĪŖAµćµÄ°×É«¹ĢĢå1/10£¬ĻņĘäÖŠÖšµĪ¼ÓČė0.1mol/LHClČÜŅŗ£¬ĒėŌŚĻĀĶ¼ÖŠ»³ö²śÉśCO2ĘųĢåĢå»ż£Ø±ź×¼×“æöĻĀ£©ÓėĖł¼ÓČėµÄŃĪĖįµÄĢå»ż¹ŲĻµ”¢£ŗ


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
½«ÉĻŹöŹÕĀśNH3µÄŌ²µ×ÉÕĘæČ”ĻĀ£¬øÄ×°³ÉĶ¼ŅŅĖłŹ¾µÄ×°ÖĆ£¬½ŗĶ·µĪ¹ÜÄŚŹĀĻČŌ¤ĪüČė2 mL H2O£¬“ĖŹ±Š”ĘųĒņĻµŌŚ²£Į§¹ÜÉĻ³Ź×ŌČ»ĖɳŚ×“Ģ¬£»½«µĪ¹ÜÄŚµÄĖ®ĀżĀżµĪČėÉÕĘæÖŠ£¬ĒįĒį»Ī¶ÆÉÕĘ棬Ķعż¹Ū²ģŹµŃéĻÖĻó±ćæÉŅŌŃéÖ¤NH3µÄijøöŠŌÖŹ”£°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)֊ѧ»Æѧ½Ģ²ÄÖŠŹĒĶعż¹ĢĢåĪļÖŹ¼ä¼ÓČČ·“Ó¦Ą“ÖĘČ”NH3µÄ£¬øĆ»Æѧ·½³ĢŹ½ĪŖ£ŗ
________________________________________________________________ӣ
(2)ĻĀĆęŹĒijĶ¬Ń§¹ŲÓŚĶõĄĻŹ¦ÄÜÓĆĶ¼¼×ÖĘČ”NH3µÄŌŅņ·ÖĪö£¬ÓŠµĄĄķµÄŹĒ_________________”£
¢ŁŌŚNH3”¤H2OÖŠÓŠĘ½ŗāNH3+H2O![]() NH3”¤H2O
NH3”¤H2O![]()
![]() +OH-£¬NaOHŹ¹Ę½ŗāĻņ×óŅʶÆ
+OH-£¬NaOHŹ¹Ę½ŗāĻņ×óŅʶÆ
¢ŚŌŚNH3”¤H2OÖŠÓŠĘ½ŗāNH3+H2O![]() NH3”¤H2O
NH3”¤H2O![]()
![]() +OH-£¬NH4ClŹ¹Ę½ŗāĻņ×óŅʶÆ
+OH-£¬NH4ClŹ¹Ę½ŗāĻņ×óŅʶÆ
¢ŪNaOHČÜÓŚĖ®Ź±·ÅČČ£¬Ź¹ĢåĻµµÄĪĀ¶ČÉżøߣ¬NH3µÄČܽā¶Č¼õŠ”
¢ÜNH4ClÓėNaOHŌŚ“ĖĒéæöĻĀæÉ·“Ӧɜ³ÉNH3£¬¼“![]() +OH-
+OH-![]() NH3ӟ+H2O
NH3ӟ+H2O
¢ŻNH4Cl»į·Ö½āŹĶ·Å³öNH3
(3)Ķ¼¼×ÖŠµÄNH4ClÓėNaOH¹ĢĢå»ģŗĻĪļÄÜ·ńÓĆCaO¹ĢĢå“śĢę__________(Ģī”°ÄÜ”±Óė”°²»ÄÜ”±)
(4)ČēŗĪÅŠ¶ĻĶ¼¼×ÖŠÉÕĘæŅŃŹÕĀśNH3? _______________________________________________”£
(5)Ķ¼ŅŅÖŠ½ŗĶ·µĪ¹ÜÖŠµÄĖ®¼·ČėÉÕĘæŗ󣬹Ū²ģµ½µÄĻÖĻóŹĒ_______________________________£¬ĖüĖµĆ÷ĮĖNH3________________________________________________________”£
¢ņ.ČēĶ¼ĖłŹ¾£ŗŌŚB²Ū֊װӊ500 mLĖ®£¬ČŻ»żĪŖa mLµÄŹŌ¹ÜA³äĀśĮĖNO2ŗĶNOµÄ»ģŗĻĘųĢå(±ź×¼×“æö)£¬½«ŹŌ¹ÜAµ¹²åČėB²ŪµÄĖ®ÖŠ”£³ä·Ö·“Ó¦ŗó£¬ŹŌ¹ÜAÖŠÓąĻĀĘųĢåµÄĢå»żĪŖ0.

Ķعżµ¼Ęų¹ÜCĶłÓąĻĀ0.5a mLĘųĢåµÄŹŌ¹ÜAÖŠĶØČėŃõĘų£¬µ±ŹŌ¹ÜAÖŠ³äĀśŅŗĢåŹ±Ķ£Ö¹ĶØČėŃõĘų£¬Ōņ¹²ĶØČėŃõĘųµÄĢå»żĪŖ__________mL£¬Č»ŗó½«ŹŌ¹ÜČ”³öĖ®²Ū£¬Ė®²ŪBÖŠČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ_________mol”¤L-1(ÉčČÜŅŗµÄĢå»żČŌĪŖ500 mL)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com