
��֪�ɶ�����Ԫ����ɵ�A��B��C��D���ֻ���������ԭ����Ŀ����Ϊ2��3��4��5������A��B��C������18�����ӣ�D����10�����ӡ���ش�
��1��A��B��D�Ļ�ѧʽ�ֱ���A ��B ��D
��2����֪8gD��O2��ȫ��Ӧ�������ȶ�������ʱ�ų�445kJ��������д����Ӧ���Ȼ�ѧ����ʽ ��
��3����CΪ��һ�͵�������Ԫ����ɵĻ����C�ڿ������ܹ���ȼ�������ֳ����������д����ѧ����ʽ ��
��CΪ��һ�͵ڶ�����Ԫ����ɵĻ������
��C����Һ�м��������Ķ������̣�����ɫ�������ɣ�д����ѧ����ʽ
��C����Һ�м��������������̺�ϡ���ᣬ�����������ܽ�����Mn2+����Ӧ�ж��������� ��
�۵�C�ֱ����ٺ͢�������Ӧʱ��������1molC��ȫ��Ӧ���ٺ͢�������Ӧ��ת�Ƶ�����֮���ǣ� ��
��1��HCl��H2S��CH4����1�֣���3�֣�
��2��CH4��g��+2O2��g��=CO2(g)+2H2O(l); ��H=-890kJ��mol-1��2�֣�
��3��2PH3+4O2 P2O5+3H2O
��2H2O2 2H2O+O2����2�֣�
2H2O+O2����2�֣�
����������2�֣�
��1��2��2�֣�
�������⿼��ԭ�ӽṹ��Ԫ��������֪ʶ������ؼ�������ǰ20��Ԫ��ԭ�ӽṹ����1���ɶ�������ԭ����ɵ�18���ӵĻ�����ΪHCl����ԭ����ɵ�18���ӵĻ�����ΪH2S����ԭ����ɵ�18���ӻ�����ΪPH3��H2O2���ɶ�������ԭ����ɵ�10���ӵĻ�����ΪCH4����2����������1molCH4��ȫȼ�շų�����Ϊ����H=-890kJ��mol-1�����䷴Ӧ���Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2(g)+2H2O(l); ��H=-890kJ��mol-1����3����CΪ��һ�͵�������Ԫ����ɵĻ������CΪPH3���������⣬ΪPH3��������ȼ���������������TΪP2O5��H2O����Ӧ����ʽΪ��2PH3+4O2 P2O5+3H2O����CΪ��һ�͵ڶ�����Ԫ����ɵĻ������CΪH2O2��H2O2�ڶ������������»�Ѹ�ٲ���������2H2O2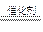 2H2O+O2����H2O2��Һ�м��������������̺�ϡ���ᣬ�����������ܽ�����Mn2+����Ԫ�ػ��ϼ۽��ͣ�������������������2H2O2
2H2O+O2����H2O2��Һ�м��������������̺�ϡ���ᣬ�����������ܽ�����Mn2+����Ԫ�ػ��ϼ۽��ͣ�������������������2H2O2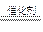 2H2O+O2��1molH2O2��ȫ��Ӧ��ת�Ƶ���1mol��H2O2��MnO2��H2SO4=MnSO4��2H2O��O2��,1molH2O2��ȫ��Ӧ��ת�Ƶ���2mol��������Ӧ��ת�Ƶ�����֮����1��2��
2H2O+O2��1molH2O2��ȫ��Ӧ��ת�Ƶ���1mol��H2O2��MnO2��H2SO4=MnSO4��2H2O��O2��,1molH2O2��ȫ��Ӧ��ת�Ƶ���2mol��������Ӧ��ת�Ƶ�����֮����1��2��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�̽��и����ڶ��νο��Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣���֪�ɶ�����Ԫ����ɵ�A��B��C��D���ֻ���������ԭ����Ŀ����Ϊ2��3��4��5������A��B��C����18�����ӣ�D����10�����ӡ���ش�
��1��A��B��D�Ļ�ѧʽ�ֱ���A ��B ��D
��2����֪8gD��O2��ȫ��Ӧ�������ȶ�������ʱ�ų�445kJ��������д����Ӧ���Ȼ�ѧ����ʽ
��3����CΪ��һ�͵ڶ�����Ԫ����ɵĻ������C����Һ�м��������Ķ������̣�����ɫ�������ɣ�д����ѧ����ʽ
��C����Һ�м��������������̺�ϡ���ᣬ�����������ܽ�����Mn2+����Ӧ�ж��������� ��
�۵�C�ֱ����ٺ͢�������Ӧʱ��������1molC��ȫ��Ӧ���ٺ͢�������Ӧ��ת�Ƶ�����֮���ǣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com