
����Ŀ��[��ѧ����ѡ��5���л���ѧ����]
�ײ������ᣬ�����Ǽ������ᣬ�ھ�ϸ�����������Ź㷺����;����ϳ�·�����£�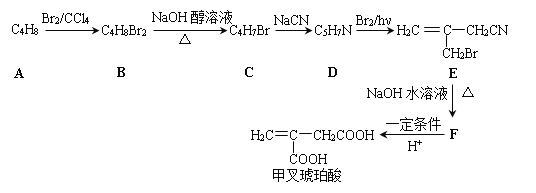
��֪��![]()
�ش��������⣺
��1���ײ��������й����ŵ�����Ϊ_______��_______��
��2��A������Ϊ_______��A��B�Ļ�ѧ����ʽΪ_______��
��3��B��C�Ļ�ѧ����ʽΪ_______��C��D�ķ�Ӧ����Ϊ_______��
��4��F�Ľṹ��ʽΪ_______��
��5���ײ��������ͬ���칹��MҲ�Dz����Ͷ�Ԫ���ᣬ��M���� _______�֣������������칹�������к˴Ź���������ʾ����壬�ҷ����֮��Ϊ3��1��2�Ľṹ��ʽΪ _______��
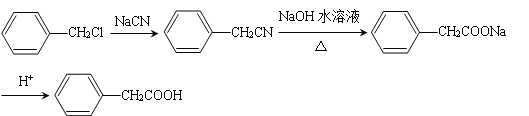
��6������������Ϣ��д����һ�ȼױ���![]() CH2Cl��Ϊԭ�ϣ����Լ���ѡ���Ʊ������ᣨ
CH2Cl��Ϊԭ�ϣ����Լ���ѡ���Ʊ������ᣨ![]() CH2COOH���ĺϳ�·��_______��
CH2COOH���ĺϳ�·��_______��
���𰸡��Ȼ� ̼̼˫�� 2-����ϩ ![]() +Br2
+Br2![]()
 +NaOH
+NaOH![]()
 +NaBr+H2O ȡ����Ӧ
+NaBr+H2O ȡ����Ӧ 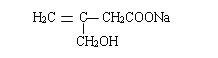 4 CH3CH=C(COOH)2
4 CH3CH=C(COOH)2 ![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
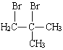
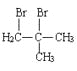
A�ķ���ʽΪC4H8�����巴Ӧ�õ�B��B������ȥ��Ӧ�õ�C��C����NaCN��Ӧ�������Ϣ�ڿ�֪C�л�����Brԭ�ӣ���AΪϩ������B��ֻ��1��Brԭ�ӿ��Է�����ȥ��Ӧ������֪AΪ![]() ��BΪ
��BΪ ��CΪ
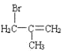
��CΪ ��DΪ
��DΪ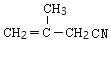 �������Ϣ�ܼ��ײ�������Ľṹ����֪D�м���Hԭ����ȫ������ԭ��ȡ������E����EΪ
�������Ϣ�ܼ��ײ�������Ľṹ����֪D�м���Hԭ����ȫ������ԭ��ȡ������E����EΪ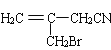 ��FΪ
��FΪ![]() ��F�ữˮ��õ��ײ������ᡣ
��F�ữˮ��õ��ײ������ᡣ
��1�����ݼײ�������ṹ��֪�����й����ŵ�����Ϊ�Ȼ���̼̼˫�����ʴ�Ϊ���Ȼ���̼̼˫����
��2��A�ܺ��巢���ӳɷ�Ӧ��A�к���˫��������E����˵��������3��̼ԭ�ӣ���A������Ϊ2-����ϩ�������ˮ�����ӳɷ�Ӧ�ķ���ʽΪ��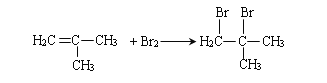 ���ʴ�Ϊ��2-����ϩ��
���ʴ�Ϊ��2-����ϩ��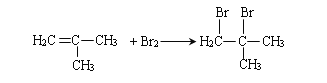 ��
��
��3���������֪B��C�Ƿ�����ȥ��Ӧ����˫�����仯ѧ����ʽΪ  +NaOH
+NaOH![]()
 +NaBr+H2O��C��D�ķ�Ӧ���������ȡ����ԭ������ȡ����Ӧ���ʴ�Ϊ��
+NaBr+H2O��C��D�ķ�Ӧ���������ȡ����ԭ������ȡ����Ӧ���ʴ�Ϊ�� +NaOH
+NaOH![]()
 +NaBr+H2O��ȡ����Ӧ��
+NaBr+H2O��ȡ����Ӧ��
��4������������Ϣ��֪��F���������������ɼײ������ᣬF��Ϊ�����ƣ�F�Ľṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5���ײ��������ͬ���칹��MҲ�Dz����Ͷ�Ԫ���ᣬ��M����4�֣����к˴Ź���������ʾ����壬�ҷ����֮��Ϊ3��1��2�Ľṹ��ʽΪ��CH3CH=C(COOH)2���ʴ�Ϊ��4��CH3CH=C(COOH)2��
��6�������軯�Ʒ�Ӧ����  ��Ȼ������������ˮ��Һ��������
��Ȼ������������ˮ��Һ��������  ���������������������
��������������������� ![]() ������
������ ��
��


 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K3[Fe(C2O4)3]��3H2O�����������(��)��ؾ��塳���Ʊ������ͻ�������������Ҫԭ�ϣ�Ҳ��һЩ�л���Ӧ�ܺõĴ�������ͼ����ʵ�����Ʊ�K3[Fe(C2O4)3]��3H2O�����̣�
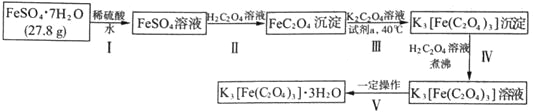
�ش��������⣺
��1������I�м���ϡ�����Ŀ����_______________��
����II�з�������Ҫ��ѧ��ӦΪһ������̣������ӷ���ʽΪ_______________��
�����£��÷�Ӧ��ƽ�ⳣ��K=_______________[��֪�����£�Ka1(H2C2O4)=5.6��10-2��Ka2(H2C2O4)=5.4��10-5��Ksp(FeC2O4)=2.1��10-7]
��2�������Լ����ɽ�FeC2O4����ΪK3[Fe(C2O4)3]����������Ϊ���Լ�a������_______________(�����)
a����ˮb������KMnO4��Һc��H2O2��Һd��ϡ����
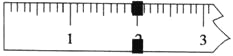
��3��ʹ��������ƽ�����Ƶõ�K3[Fe(C2O4)3]��3H2O����������ƽƽ��ʱ�������������������Ϊ20 g������ʾ����ͼ��ʾ�����Ƶþ��������Ϊ___________g����ʵ����K3[Fe(C2O4)3]��3H2O�IJ���Ϊ_______________��(��֪�� K3[Fe(C2O4)3]��3H2O����Է�������Ϊ491��FeSO4��7H2O����Է�������Ϊ278)
��4��ij�о�С�齫K3[Fe(C2O4)3����һ�������¼��ȷֽ��������ͼ��ʾװ��(���ظ�ʹ��)ȷ�����ú�̼Ԫ�ص��������ΪCO��CO2��
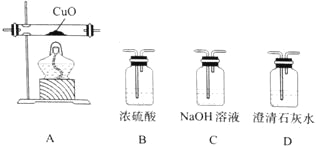
�����������ҵķ���װ�õ�����˳��Ϊ_______________(��װ�����) ��ȷ����������к�CO������Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ժ��ܷϴ���(��Ҫ�ɷ�ΪCo��Fe��SiO2)Ϊԭ������ȡ������(Co2O3)�����ҹ����������ܳ����Ĺ�ҵ����֮һ���������������£�
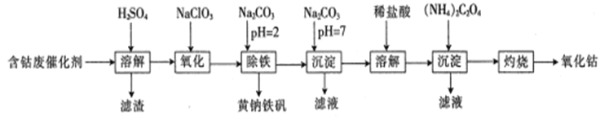
��֪��KMnO4��������ǿ����������HBr��HCl��Fe2���ȡ�
��ش��������⣺
��1�����ܽ⡱ǰ���轫���ܷϴ��������Ŀ����____________�����ܽ⡱��������������Ҫ�ɷ���________________(�ѧʽ)��
��2������������Ŀ���ǽ�Fe2��������Fe3�����÷�Ӧ�����ӷ���ʽΪ__________��ʵ�����ܷ�ѡ��KMnO4��Һ����Fe2���Ƿ���ȫ����?______ (��ܡ����ܡ�)��������______________________________��
��3���������������У���Fe3����CO32�������ʵ������ʱ��ǡ����ȫ��Ӧ���ɻ�������[Na2Fe6(SO4)4(OH)12]������д���÷�Ӧ�Ļ�ѧ����ʽ��_____________
��4������Na2CO3��Һ�����ɡ������������˺�����ˮϴ�ӳ��������������Ѿ�ϴ�Ӹɾ��ķ�����______________
��5����ȡ10.0g���ܷϴ���(���ܵ���������Ϊ70.8%)ģ�������������̽���ʵ�飬���յõ�7.47g�����ܣ����ʵ��IJ���Ϊ__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڻ�ƿ�м������ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
�ɷ� | ������g�� | Ħ��������g ��mol-1�� |
���� | 25��00 | 342 |
����� | 0��25 | 174 |
��˾ƥ�� | 0��17 | 180 |
������� | 0��25 | 158 |
������ | 0��02 | 170 |
��1���������ʻ����ʼ����ijɷ��У����ڷǵ���ʵ���________��
A������ B������� C��������� D��������
��2�����ʻ����ʼ�����K+����˾ƥ���в���K+�������ʵ���Ũ��Ϊ_______ mol/L����ע�⣺ֻҪ����ԭʼ����д����ʽ������Ҫ��������㣩
��3�������������ʻ����ʼ�������������У��ձ���������ƽ��ҩ�ס�________________��______________��_______________�����ں�������д��ȱ���������ƣ�
��4������Һ���ƹ����У����в��������ƽ��û��Ӱ�����___________��
A������ʱ��������ƿ�̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�NaCl��Һ��δϴ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ȼ�շ��ⶨij�����ᣨCxHyNzOp���ķ�����ɡ�ȡm g ���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2������ͼ��ʾװ�ý���ʵ�顣

��ش��������⣺
��1��ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������__________________
��2������װ������Ҫ���ȵ�������_________________������ĸ��գ���ͬ��������ʱӦ�ȵ�ȼ_______���ľƾ��ơ�
��3��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��_____________________��
��4��װ��D��������___________________________��
��5����ȡN2�����ʱ��Ӧע��
��______________________
��______________________
��______________________
��6��ʵ���в��N2�����ΪV mL����״������Ϊȷ���˰�����Ļ�ѧʽ������Ҫ���й�������_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�ֺ���Ҫ�Ľ��������Է���һϵ�з�Ӧ�Ʊ����ʣ���ͼ��ʾ������˵���������
�� ��

A.��Ӧ���ֳ����ȷ�Ӧ��������Ұ�⺸������
B.��Ӧ�ڢ۶����������ɣ���������������ʱת�Ƶĵ��������
C.��ҵ���÷�Ӧ���Ʊ���ʱ�����������ʯ�Խ����������������¶�
D.���÷�Ӧ���Ʊ�Al(OH)3����������Al2(SO4)3��Һ�еμ�������NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС����ݷ�Ӧ2KMnO4��10FeSO4��8H2SO4=2MnSO4��5Fe2(SO4)3��K2SO4��8H2O�������ԭ��أ����мס������ձ��и����ʵ����ʵ���Ũ�Ⱦ�Ϊ1 mol��L��1����Һ�������Ϊ200 mL��������װ�б���K2SO4��Һ��
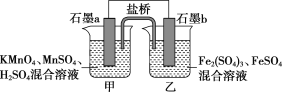
�ش��������⣺
��1����ԭ��ص�������ʯī________(����a������b��)������________��Ӧ��
��2����ع���ʱ�������е�SO42-����________(����������������)�ձ���
��3�����ձ��еĵ缫��Ӧʽ�ֱ�Ϊ
��__________________________________________________________________��
��________________________________________________________________��
��4������������Һ������仯��MnSO4Ũ�ȱ�Ϊ1.5 mol��L��1����Ӧ��ת�Ƶĵ���Ϊ____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ����������������ء��밴Ҫ�ش��������⣺
��1��K2FeO4��һ����Ҫ�ľ�ˮ�����������з����Ƶã�2Fe(OH)3 + 3Cl2 +10KOH![]() 2 K2FeO4 + 6KCl +8H2O�ڸ÷�Ӧ�У���ԭ����____________������Ӧ����1mol K2FeO4����ʱ�����ĵ�Cl2�ڱ���µ����Ϊ____________L��ת�Ƶ��ӵ���ĿΪ____________��
2 K2FeO4 + 6KCl +8H2O�ڸ÷�Ӧ�У���ԭ����____________������Ӧ����1mol K2FeO4����ʱ�����ĵ�Cl2�ڱ���µ����Ϊ____________L��ת�Ƶ��ӵ���ĿΪ____________��
��2���Ա���ù�����������������д�����������������̼��Ӧ�Ļ�ѧ����ʽ����˫���ŷ���ʾ����ת�Ƶķ������Ŀ��_____________________________________________��
��3��������С�մ���������ķ��ݼ���д��С�մ�����ˮ�ĵ��뷽��ʽ��_____��
��4����ҵ����Ư�۵Ļ�ѧ����ʽΪ________________________________��������ˮ�����������²�����ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ��_________________________��
��5��KAl(SO4)2��12H2O��������ˮ����_____(��ѧʽ)�����������ʣ��Ӷ��ﵽ��ˮ���á�
��6��������ˮ��Һ������Ϊ__________������Ϊľ��_______________��ԭ�ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2008�꣬��¹�ȶ������Ʒ��ҵΪʹ�����ʺ������ϸ�����������谷��ʹ������ͯ������ʯ��ʵ���ҿ�������ʵ��װ�òⶨ�����谷�ķ���ʽ��

��֪�����谷����Է�������Ϊ126��ȡ1.26 g�����谷��Ʒ�����ڴ����г��ȼ�գ����ɶ�����̼��ˮ��������ʵ����װ��B����0.54g��C����1.32g������F��ˮ�����Ϊ672 mL(�ɰ���״������)��
��1��Eװ�õ�������_____��
��2����Ҫ���ȵ�װ����____(����ĸ����)��
��3��װ��D��������_____��
��4��F������ʱӦ��ע�����____��_____��
��5�������谷�ķ���ʽΪ____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com