(7��)��֪��CH3CH2OH+NaBr+H2SO4(Ũ)  CH3CH2Br+NaHSO4 +H2O��
CH3CH2Br+NaHSO4 +H2O��
ʵ�����Ʊ������飨�е�Ϊ38.4�棩��װ�úͲ������£�

�ٰ���ͼ��ʾ�������������װ�õ������ԣ�Ȼ����U�ιܺʹ��ձ�������ˮ������Բ����ƿ�м���10mL95���Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ����С����ȣ�ʹ���ַ�Ӧ��
�Իش��������⣺
(1)��Ӧʱ���¶ȹ��߿ɿ����к���ɫ���������������Ļ�ѧʽΪ
��
(2)Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
(3)��Ӧ������U�ι��д��Ƶ���������ػ�ɫ����U�ι��еĻ���ﵹ���Һ©���У����ã���Һ��ֲ��Һ��ȡ (��ϲ㡱���²㡱)Һ�塣Ϊ�˳�ȥ���е����ʣ����ѡ�������Լ��е� (�����)��
| A��Na2SO3��Һ | B��H2O | C��NaOH��Һ | D��CCl4 |
(4)Ҫ��һ���Ƶô�����C
2H
5Br��������ˮϴ��Ȼ�������ˮCaCl
2����ٽ���
(���������)��
(5)���м���ʵ�鲽�裬�����ڼ����������е���Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��
(�����)��
�ټ��� �ڼ���AgNO
3��Һ �ۼ���ϡHNO
3�ữ �ܼ���NaOH��Һ ����ȴ

 CH3CH2Br+NaHSO4 +H2O��
CH3CH2Br+NaHSO4 +H2O��
 ����������������ϵ�д�
����������������ϵ�д�
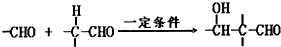
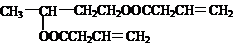
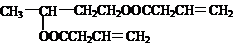
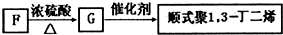
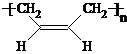
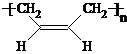















 ��R��R'������������ԭ�ӣ�
��R��R'������������ԭ�ӣ�






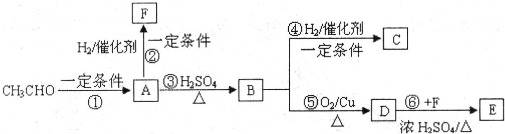
 ���ĺϳ�·�ߣ�
���ĺϳ�·�ߣ�



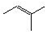 �����о����֣��Ҳϵ���Ϣ��Ϊ��CH3��CH2��2CH=CH-CH=CH��CH2��8CH3�����ϵ���Ϣ���У�
�����о����֣��Ҳϵ���Ϣ��Ϊ��CH3��CH2��2CH=CH-CH=CH��CH2��8CH3�����ϵ���Ϣ���У�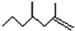 ���ף���
���ף���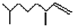 ���ң�������˵����ȷ���ǣ�������
���ң�������˵����ȷ���ǣ�������