
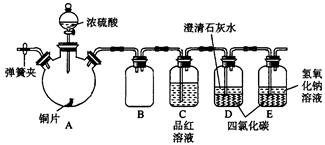
 CuSO4+SO2��+H2O��2�֣� ��2����ֹ������2�֣�
CuSO4+SO2��+H2O��2�֣� ��2����ֹ������2�֣�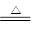 CuSO4+SO2��+H2O��
CuSO4+SO2��+H2O��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B��ֻ�Т� | C��ֻ�Т� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
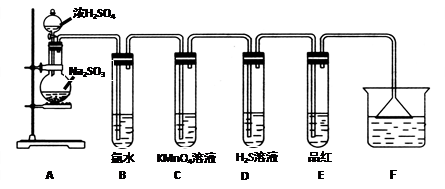
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����>��>��>�� | B����>��>��>�� |
| C����>��>��>�� | D����>��>��>�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ͨ��ŨH2SO4�� | B��ϡH2SO4�������������� |
| C��ŨH2SO4��C2H5OH���ȵ�170�� | D����ʪ������ͨ��ʢ��ŨH2SO4��ϴ��ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
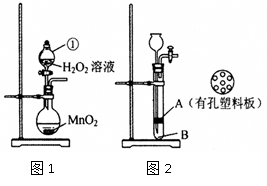
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʵ����� | �� | �� | �� | �� |
| ������Һ�������mL�� | 50 | 50 | 50 | 50 |
| �������g�� | 9.260 | 13.890 | 20.835 | 32.410 |
| ��������������mL�� | 1344 | 2016 | 3024 | 2464 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
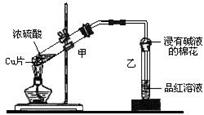
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
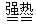 Fe2O3+SO2��+ SO3��+ 14H2O
Fe2O3+SO2��+ SO3��+ 14H2O| A����BaSO4���� | B����BaSO3���� |
| C��ͬʱ��BaSO4��BaSO3���� | D����SO3�ݳ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com