


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| 11200 |
| V |
| 11200 |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ����� | ʵ������ |
| ���ɼУ�ͨ��һ��ʱ��CO2���رյ��ɼУ� | |
| ��Һ©����������Ũ���Ỻ��������ƿ�У��رջ����� | ���������� |
| ������ƿ����Ӧ��ʼ��ֹͣ���ȣ� | ��A���к���ɫ���������һ��ʱ���������ɫ��dz�� B����Һ����ɫ�� C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��A������ʣ�࣮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���������� | ���ͻ���� |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� | �������ۻ��������������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� | ����Ӧ���Ũ�ȣ���ѧƽ��������Ӧ�����ƶ� |
| C��֤��Mg��OH��2��������ת��ΪFe��OH��3 ���� |
��2mL 1mol/L NaOH��Һ���ȼ���3��1mol/L MgCl2��Һ���ټ���3��1mol/L FeCl3��Һ |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� | ����Һ��һ�����д�����SO42- |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� |
| C��֤��Mg��OH��2��������ת��ΪFe��OH��3���� |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������и�����ҵ��������һ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ������������Ӧԭ���Ľ��͡����۾���ȷ��һ����
���������� ���ͻ����
A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� �������ۻ���������������
B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� ����Ӧ���Ũ�ȣ���ѧƽ��������Ӧ�����ƶ�
C���������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ����Һ�����ȣ�δ����ɫ���� ����δˮ��
D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� ����Һ��һ�����д�����SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(��)����ʵ��������ʵ����ʵ��������ȷ���� ������ţ���
�� ʵ���������Ȼ�������Һʱ�����Ȼ��������ܽ��������У�Ȼ��������ˮϡ�Ͳ������������ۡ�
�� ����һ��Ũ�ȵ���Һʱ����������ƿ�Ŀ̶��ߣ���ʹ���Ƶ�Ũ��ƫ�ߣ�ʵ���Ҳⶨ�к���ʱ�����������ʹ�ⶨ���ƫ�͡�
�� ��Fe2(SO4)3��Һ�����������ɲ����գ����õ�����ɫ��ĩ
�� ʵ������ͭƬ��ϡ���ᷴӦ���������������ˮ���ռ�
�� �Թ��м����������ۣ��ټ���һ����ϡ���ᣬ����3��4���ӣ�Ȼ�����������Һ��Ƭ�̺�ܱ����С�����������
�� ��ˮ�еμ�Al2(SO4)3��Һ����Al2(SO4)3��Һ�еμӰ�ˮ������ͬ
�� ������ˮ�����۵Ĵ��������Ʊ��屽
�� �ֱ��������pH����ͬ������ʹ����еμӵ�Ũ�ȵ�����������Һ����ȫ�к�ʱ���ĵ�����������Һ�����һ����
��.����ѧ��ѧʵ���У�ͨ������ˮ����ͭ����������ˮ�Ĵ��ڡ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������á�
�����ף�ȡ2ҩ��ϸС������ͭ��������_______��������������������������______����С���������Ȳ��ò�������ͣ���裬�����������___________�н�����ȴ����ѡ�ú���������գ��������в����ձ���ͨ������ԹܼС��������������ǣ���
�����ң�ȡ2ҩ�����������ͭ������С�ձ��У�����20 mLŨ���ᣨ��������������98%�������ò��������裬����5 min����ȥŨ���ᣬ����ˮ�Ҵ�ϴ�����Σ�������ֽ�����ɡ�
�����������ۡ�
��1���������У������¶��Ը�ʱ����ֱ������ԭ����_________���û�ѧ����ʽ��ʾ����
��2���������У�Ũ�����������__________��Ϊ�˲��˷�ҩƷ������ˮ�Ҵ�ϴ��Һ���������ķ�����______ �����õ���Ҫ������������ƿ����Һ�ܡ���ƿ���ƾ���________________�ȡ�
��3�����Ƶõ���ˮ����ͭ����ij˫��ˮ���Ƿ�ˮʱ�����˷��ֹ�������⣬�����ָ�˫��ˮ�������ݲ������Դ����кβ���______________��
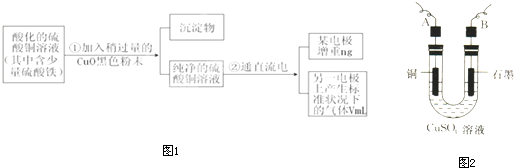
 ��4��Ŀǰ��ҵ��������̽����ŨHNO3������������Cu��ŨH2SO4��ŨHNO3��Ӧ����ȡ��Ъ���ȡ�����ŨHNO3�ķ������Ʊ�CuSO4•5H2O���¹��ա�ģ���Ʊ�װ������ͼ��ʾ��
��4��Ŀǰ��ҵ��������̽����ŨHNO3������������Cu��ŨH2SO4��ŨHNO3��Ӧ����ȡ��Ъ���ȡ�����ŨHNO3�ķ������Ʊ�CuSO4•5H2O���¹��ա�ģ���Ʊ�װ������ͼ��ʾ��
������һ����ͼװ���У���Һ©����װ��Һ����________����Ӧ����ʱ����ȡCuSO4•5H2O�IJ�����������________________�����Ƚ�����ƿ�е�Һ�嵹���ձ�����ȴ����������CuSO4?5H2O �����ˡ����ɡ�
�����������ҵ����ʯ��������β�������˷�ֹ������Ⱦ�⣬���ܵõ����о���ʵ�ü�ֵ�ĸ���Ʒ�D�D ������ơ�β����������������ƵĻ�ѧ����ʽ��_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com