
��14�֣���ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����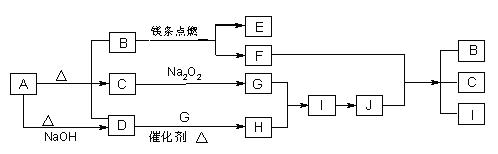
����д���¿հף�
��1��A�Ļ�ѧʽ B�ĵ���ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D��G�� H ��
F+J �� B + C + I ��
��3��0��3mol I������C��Ӧת�Ƶ��ӵ����ʵ���Ϊ_________________mol��
��4���ݻ�Ϊ10 mL���Թ��г���I��G�Ļ�����壬������ʢˮ��ˮ���У�ˮȫ�������Թܣ���ԭ���������I��G������ֱ�Ϊ_____mL��_____mL��
 Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����

����д���¿հף�
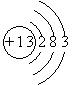
��1��A�Ļ�ѧʽ B�ĵ���ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D��G�� H ��
F+J �� B + C + I ��
��3��0��3mol I������C��Ӧת�Ƶ��ӵ����ʵ���Ϊ_________________mol��
��4���ݻ�Ϊ10 mL���Թ��г���I��G�Ļ�����壬������ʢˮ��ˮ���У�ˮȫ�������Թܣ���ԭ���������I��G������ֱ�Ϊ_____mL��_____mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��15�֣���ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����
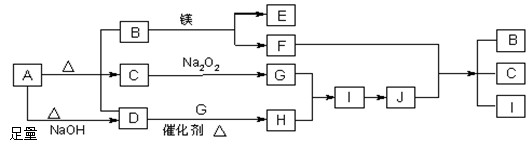
����д���¿հף�
��1��A�Ļ�ѧʽ B�ĵ���ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D��G�� H ��
F+J �� B + C + I ��
��3��д�����ϣ�A+NaOH��D�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������У2010��ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��14�֣���ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����
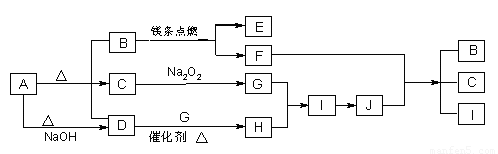
����д���¿հף�
��1��A�Ļ�ѧʽ B�ĵ���ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
D��G�� H ��
F+J �� B + C + I ��
��3��0��3mol I������C��Ӧת�Ƶ��ӵ����ʵ���Ϊ_________________mol��
��4���ݻ�Ϊ10 mL���Թ��г���I��G�Ļ�����壬������ʢˮ��ˮ���У�ˮȫ�������Թܣ���ԭ���������I��G������ֱ�Ϊ_____mL��_____mL��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com