
A��B��C���Ƕ�����Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��B��C����Ԫ�ص�ԭ��������֮��Ϊ32��DԪ��ԭ�ӵ�����������Ϊ�������������2����������˵����ȷ���� ![]() ѧ����
ѧ����
![]()
![]() ѧ����
ѧ����
A��Ԫ��D�γɵ���̬�⻯��һ�������������η���![]() ѧ����
ѧ����
B��A��B����Ԫ�ص���̬�⻯����������Ƕ�Ӧ������������Ӧ��ˮ�������Ӧ���ҷ�Ӧ������ͬ ![]() ѧ����
ѧ����
C��B��C����Ԫ�ؿ��γ�BC6�ͻ�����û������ڿ����в���ȼ��![]() ѧ����
ѧ����
D����֪BC6�Ľṹ���������幹�ͣ���C�������ȶ���ͬλ�أ���BC6�IJ�ͬ����������10��![]() ѧ����
ѧ����
CD
A��B��C��D����Ԫ������Ϊ����������̼��̼Ԫ������ڶ�����̬������һ�������������η��ӣ�NH3��HNO3��H2S��ŨH2SO4�ֱ�Ӧʱ��ǰ���Ƿ�������ԭ��Ӧ��������������ԭ��Ӧ����A��B���������SF6��S��+6�ۣ������ٱ���������1��F�Ļ�ԭ�Ժ��������ܱ�O2��������Cѡ����ȷ�����������У������ľ���ֻ�����֣������е�����λ�ü��Խ���λ�ã���D��Ҳ��ȷ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

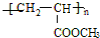
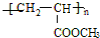
| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


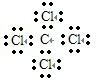

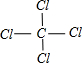
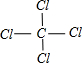
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
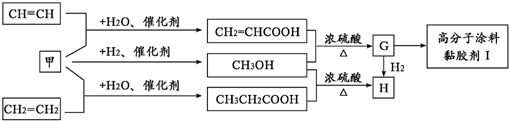


| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��֪C�ĺ˵��������A��B��Ԫ��ԭ�Ӻ��������֮�ͣ���д��A��BԪ���γɻ�����ĵ���ʽ��___________��д��B��CԪ�ؿ����γɵ����ֻ�����Ľṹʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ѡ����
A��B��C���Ƕ�����Ԫ�أ�ԭ��������������B�ǵؿ��к�������Ԫ�أ�������AxBy�ж�����ʽ���еĿ��Ե������ꣻ������CmBnҲ�в�ͬ��ʽ������m��n������2��1��1��1�������ж���ȷ���ǣ�
A��ԭ�Ӱ뾶�ɴ�С��˳���ǣ�C>B>A
B��AxBy��A�Ļ��ϼ۲�����Ϊ+1��
C��C��B�γɵĻ���������ˮ���ܵõ�����B
D��A��B��Ԫ�ص���̬�⻯��������Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com