
(10��)ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ,��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
�Իش��������⣺
(1)ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ���(����)����________����________����________����________��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D�����Ը��������Һ
(2)��˵��SO2���ڵ�������______________________________________________��
(3)ʹ��װ�âڵ�Ŀ����________________________________________________��
(4)ʹ��װ�â۵�Ŀ����__________________________________________________��
(5)ȷ֤��ϩ���ڵ�������__________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)ʵ�������Ҵ���Ũ���Ṳ����ȡ��ϩ�������¶ȹ�����������SO�����������������ͼ��ʾʵ�飬����֤��������������Ƿ�����ϩ��SO2��
��1��װ����X�����������ǣ� �����Ƭ�������ǣ� ��
��2��д��������ϩ�ķ�Ӧ����ʽ�� ��
��3��A��B��C��Dװ���п�ʢ�ŵ��Լ���(�����������Լ���ѡ���������)��
��Ʒ����Һ����NaOH��Һ������ˮ����KMnO��������Һ��
A�� ��B�� ��C�� ��D�� ��
��4����˵��SO��������ڵ������� ��
��5��ȷ֤������ϩ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�����и߶���ѧ����ĩ���Ի�ѧѡ��ģ�飨5������ ���ͣ�ʵ����
(10��)ʵ�������Ҵ���Ũ���Ṳ����ȡ��ϩ�������¶ȹ�����������SO�����������������ͼ��ʾʵ�飬����֤��������������Ƿ�����ϩ��SO2��
��1��װ����X�����������ǣ� �����Ƭ�������ǣ� ��
��2��д��������ϩ�ķ�Ӧ����ʽ�� ��
��3��A��B��C��Dװ���п�ʢ�ŵ��Լ���(�����������Լ���ѡ���������)��
��Ʒ����Һ����NaOH��Һ������ˮ����KMnO��������Һ��
A�� ��B�� ��C�� ��D�� ��
��4����˵��SO��������ڵ������� ��
��5��ȷ֤������ϩ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��ͨ�к����ظ�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(10��)ʵ���ҳ��ö������̺�Ũ���ᷴӦ��ȡ������MnO2+4HCl��Ũ��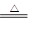 MnCl2 + Cl2��+ 2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
MnCl2 + Cl2��+ 2H2O��ijѧϰС�����ô�ԭ�������ͼ��ʾװ����ȡ������̽�������ʡ�
���ڸ�ʵ���У��ײ��ֵ�װ���� ����д��ĸ����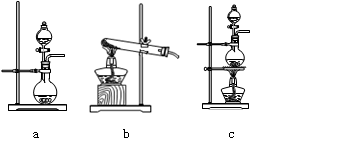
����װ���пɹ۲쵽����ɫ�仯Ϊ�� ��
�DZ�װ����FeCl2��Һ��Cl2��Ӧ�����ӷ���ʽ�� ��
�ȶ�װ����ͨ������Cl2�����Ƶ�ij�������г��õ�Ư�ס����������ʡ���֪̼�������ǿ�ڴ����ᣬ����з�����Ӧ�Ļ�ѧ����ʽ�� ��
�ɸ�ʵ��������Ե�ȱ�ݣ��Ľ��ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ����Э�����һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
(10��)ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ ,
��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
,
��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������

�Իش��������⣺
(1)ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ���(����)����________����________����________����________��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D�����Ը��������Һ
(2)��˵��SO2���ڵ�������______________________________________________��
(3)ʹ��װ�âڵ�Ŀ����________________________________________________��
(4)ʹ��װ�â۵�Ŀ����__________________________________________________��
(5)ȷ֤��ϩ���ڵ�������__________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com