

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д� ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶ȣ��棩 | 20 | 40 | 60 | 80 | 100 |
| ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
| ���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
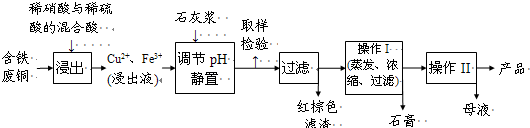
(14��) ��ӡ��ʹ�õ�ī����Ҫ�ɷ���Fe3O4����ͼ����������������Fe3O4�Ĺ��գ�
��֪���ٹ�ҵ�̷���FeSO4�ĺ���Ϊ52.5%�����е����ʲ����뷴Ӧ��
�� Fe(OH)2��2Fe(OH)3��Fe3O4��4H2O
�� 12.16�~1000�~52.5%=6384, 6384��152=42��ش��������⣺
��1�����ij����������� ��
��2��Fe3O4��ϡ���ᷴӦ�����ӷ���ʽ�� �����鷴Ӧ�����Һ�к�Fe3+�ķ��� ��
��3���ڹ��������У�ͨ������������������ʱ�Ļ�ѧ����ʽ�ǣ� ��
��4�������пɻ�õĸ���Ʒ�� ����ȡ�ø���Ʒ�IJ���˳���� ����д��ţ�
a������ b������Ũ�� c����ȴ d���ᾧ e��ϴ��
��5�������������У�����ҵ�̷���Ͷ��������12.16 kg/h��Ϊʹ��Ʒ�ϴ����������������ӦΪ L/h�����跴Ӧ�ڱ�״̬�½��У�������O2ռ20%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ�ϰ�һ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
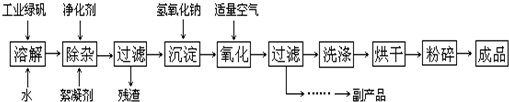
(14��). ��ӡ��ʹ�õ�ī����Ҫ�ɷ���Fe3O4����ͼ���������� ������Fe3O4�Ĺ��գ�
������Fe3O4�Ĺ��գ�
��֪���� ��ҵ�̷���FeSO4�ĺ���Ϊ52.5%�����е����ʲ����뷴Ӧ��
�� Fe(OH)2��2Fe(OH)3��Fe3O4��4H2O
�� 12.16�~1000�~52.5%=6384,6384��152=42��ش��������⣺
��1�����ij����������� ��
��2��Fe3O4��ϡ���ᷴӦ�����ӷ���ʽ�� �����鷴Ӧ�����Һ�к�Fe3+�ķ��� ��
��3���ڹ��������У�ͨ������������������ʱ�Ļ�ѧ����ʽ�ǣ� ��
��4�������пɻ�õĸ���Ʒ�� ����ȡ�ø���Ʒ�IJ���˳���� ����д��ţ�
a������ b������Ũ�� c����ȴ d���ᾧ e��ϴ��
��5�������������У�����ҵ�̷���Ͷ��������12.16 kg/h��Ϊʹ��Ʒ�ϴ����������������ӦΪ L/h�����跴Ӧ�ڱ�״̬�½��У�������O2ռ20%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(14��). ��ӡ��ʹ�õ�ī����Ҫ�ɷ���Fe3O4����ͼ����������������Fe3O4�Ĺ��գ�

��֪���� ��ҵ�̷���FeSO4�ĺ���Ϊ52.5%�����е����ʲ����뷴Ӧ��
�� Fe(OH)2��2Fe(OH)3��Fe3O4��4H2O
�� 12.16�~1000�~52.5%=6384, 6384��152=42��ش��������⣺
��1�����ij����������� ��
��2��Fe3O4��ϡ���ᷴӦ�����ӷ���ʽ�� �����鷴Ӧ�����Һ�к�Fe3+�ķ��� ��
��3���ڹ��������У�ͨ������������������ʱ�Ļ�ѧ����ʽ�ǣ� ��
��4�������пɻ�õĸ���Ʒ�� ����ȡ�ø���Ʒ�IJ���˳���� ����д��ţ�
a������ b������Ũ�� c����ȴ d���ᾧ e��ϴ��
��5�������������У�����ҵ�̷���Ͷ��������12.16 kg/h��Ϊʹ��Ʒ�ϴ����������������ӦΪ L/h�����跴Ӧ�ڱ�״̬�½��У�������O2ռ20%����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com