
�Ȼ���ͭ��CuCl���㷺���ڻ�����ӡȾ���л��ϳɵ���ҵ��CuCl�����ڴ���ˮ��������������Ũ�Ƚϴ����ϵ���ڳ�ʪ��������ˮ���������Ժ���ͭ����Ҫ�ɷ���Cu������CuO��Ϊԭ�ϣ���������������ֽ⼼�������Ȼ���ͭ�Ĺ��չ�������ͼ��ʾ��

�ش��������⣺
��1��CuCl��CuԪ�������ڱ��е�λ��Ϊ___________��
��2���������NԪ�ر���ԭΪ��ͼۣ���Cu�ܽ�����ӷ���ʽΪ________���ܽ��¶�Ӧ������60~70�棬ԭ����______________��
��3��д�����������Ҫ��Ӧ�����ӷ���ʽ________��(NH4)2SO3Ҫ�ʵ�������Ŀ���У���֤Cu2+�Ļ�ԭ���ʣ�__________����֪NH4Cl��Cu2+�����ʵ���֮��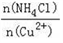 ��Cu2+�����ʵĹ�ϵ��ͼ��ʾ�����Ȼ���������ӵ�һ��Ũ�Ⱥ��Ȼ���ͭ�ij����ʼ��٣�ԭ����________��
��Cu2+�����ʵĹ�ϵ��ͼ��ʾ�����Ȼ���������ӵ�һ��Ũ�Ⱥ��Ȼ���ͭ�ij����ʼ��٣�ԭ����________��
��4����������Ҵ�ϴ�ӵ�Ŀ����__________��
��5���Ȼ���ͭ�Ķ���������
�ٳ�ȡ��Ʒ0.250g��10mL������FeCl3��Һ��250mL��ƿ�У�����ܽ⣻
����0.100mol��L��1������[Ce(SO4)2]����Һ�ⶨ����֪��CuCl+FeCl3=CuCl2+FeCl2��Fe2++Ce4+=Fe3++Ce3+��
����ƽ��ʵ�������±���ƽ��ʵ�������ܳ���1%����
ƽ��ʵ����� | 1 | 2 | 3 |
0.250g��Ʒ�������������Һ�������mL�� | 24.35 | 24.05 | 23.95 |
����Ʒ��CuCl�Ĵ���Ϊ_______���������3λ��Ч���֣���
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ʡ�߶���ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ������
�����£�ijˮ��ҺM�д��ڵ������У�Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
��1��д����H2A�ĵ��뷽��ʽ________________��
��2������ҺM��10 mL 2 mol��L-1NaHA��Һ��2 mol��L-1NaOH��Һ�������϶��ɣ�����ҺM��pH________7�����������������=��������Һ������Ũ���ɴ�С˳��Ϊ________________����֪������Ksp(BaA)��1.8��10-10����û����Һ�м���10 mL 1 mol��L-1BaCl2��Һ����Ϻ���Һ�е�Ba2��Ũ��Ϊ__________ mol��L-1��
��3������ҺM�����������������0.01 mol��L-1��H2A��Һ����0.01 mol��L-1��NaHA��Һ����0.02 mol��L-1��HCl��0.04 mol��L-1��NaHA��Һ��������Һ���������������Һ��H2A����Ũ������Ϊ________��pH�ɴ�С��˳��Ϊ_________________��
��4������ҺM��pH��3��H2A��ҺV1 mL��pH��11��NaOH��ҺV2 mL��Ϸ�Ӧ���ã������Һc(H��)/c(OH-)��104��V1��V2�Ĵ�С��ϵΪ_________������ڡ���С�ڡ������ڡ����п��ܡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ��ѧ�ڵ�һ��ģ�鿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ʵ�飺�ٳ�ȥ����ֲ�����е�ˮ�ڻ��յ��CCl4��Һ�е�CCl4����ʳ�þƾ������в�ҩ��ȡ���е���Ч�ɷ֡��������ϻ��Һ����ȷ����������
A. ��Һ����ȡ������ B. ��ȡ������Һ
C. ��Һ��������ȡ D. ������ȡ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�����и߶���ѧ����ĩ���ԣ�������ѧ�Ծ��������棩 ���ͣ�ѡ����
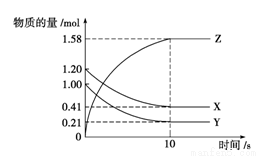
A. ��Ӧ��0��10 s�ڣ���Z��ʾ�ķ�Ӧ����Ϊ0��158 mol��L��1��s��1
B. ��Ӧ��0��10 s�ڣ�X�����ʵ���Ũ�ȼ�����0��79 mol��L��1
C. ��Ӧ���е�10 sʱ��Y��ת����Ϊ79.0%
D. ��Ӧ�Ļ�ѧ����ʽΪX(g)��Y(g)�PZ(g)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�����и߶���ѧ����ĩ���ԣ�������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ǿ������ʼ��ǵ���ʵ������ȫ��ȷ����
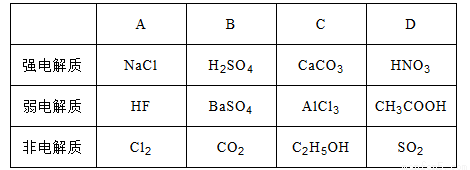
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������и�����Ӧ���¿����壩���ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼ��ʾװ�ý�������ʵ�飬a��b��c����װ�Լ����±���ʾ������ʵ����������۶�Ӧ��ϵ��ȷ��һ����
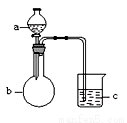
ѡ�� | a | b | c | ���� | ���� |
A | ����ʳ��ˮ | ̼���� | ��ˮ | c����Һ��ɫ��ȥ | ��Ȳ������ԭ��Ӧ |
B | Ũ���� | KMnO4���� | NaBr��Һ | c����Һ����ɫ���ɫ | Cl2�������Ա�Br2ǿ |
C | ϡ���� | ����ʯ | Na2SiO3��Һ | c���а�ɫ��״�������� | ̼������Աȹ���ǿ |
D | ���� | Na2SO3���� | ����KMnO4��Һ | c����Һ��ɫ��ȥ | SO2����Ư���� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����3�¸߿���Ӧ�Բ������ۻ�ѧ�Ծ��������棩 ���ͣ������
��ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��װ������ͼ��ʾ���г�������ȥ������Ҫ�������£�
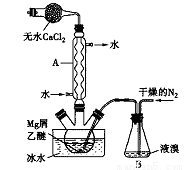
����l������ƿ��װ��10gþм��150mL��ˮ���ѣ�װ��B�м���������Һ�塣
����2������ͨ�����ĵ�����ֱ������ȫ��������ƿ�С�
����3����Ӧ��Ϻ�ָ������£����ˣ�����Һת������һ�������ƿ�У���ȴ��0�棬�������壬�ٹ��˵������Ѻ��廯þ��Ʒ��
����4���������ñ��ܽ��Ʒ����ȴ��0�棬�������壬���ˣ�ϴ�ӵ������Ѻ��廯þ��������160��ֽ����ˮMgBr2��Ʒ��
��֪����Mg��Br2��Ӧ���ҷ��ȣ�MgBr2����ǿ��ˮ�ԡ�
��MgBr2+3C2H5OC2H5= MgBr2��3C2H5OC2H5
��ش�
(1)����A��������___________������ʵ���е�������____________��
(2)����2�У����Խ�Bװ���е�����ȫ��������ƿ�е�ԭ����_______����ʵ�����������Һ��һ����ȫ����������ƿ�У�������_____________��
(3)����3�У���һ�ι��˵õ��Ĺ���������______�����ݲ���3��4�����ܽ�������Ѻ��廯þ�����е��������ʣ�_________��
(4)�����Mg���������ʵ��֤��O2�������Ա�N2��ǿ��________��
(5)������õ��IJ�Ʒ�ڸ���������ȴ�����º�����������Ϊ61.4g�����ʵ����ȡMgBr2�IJ�����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
ijƷ�ƻ�ױƷ����Ҫ�ɷ�Z��������Ч��ԭ����������ȡ���ֿ�������ͼ��ʾ��Ӧ�ϳɡ����ж�X��Y��Z����������ȷ����

A. X��Y��Z���ܺ�NaOH��Һ��Ӧ
B. X��Z���ܺ�Na2CO3��Һ��Ӧ�������ܺ�NaHCO3��Һ��Ӧ
C. Y���ܷ����Ӿ۷�Ӧ��Ҳ�ܷ������۷�Ӧ
D. Y����������ԭ�Ӳ����ܹ�ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ����ú��ˮΪԭ��ͨ��һϵ��ת���ɱ�Ϊ�����Դ������ҵԭ�ϼ״���
��1����֪��C(s)+O2(g)=CO2(g) ��H1
��2H2(g)+O2(g)=2H2O (l) ��H2
��H2O (l)= H2O (g) ��H3
��̼��ˮ������ӦC(s)+2H2O(g) CO2(g)+2H2(g)�Ħ�H =________��
CO2(g)+2H2(g)�Ħ�H =________��
��2����ҵ��Ҳ���Խ�����������Ӧ�õ���CO2��H2��һ���ϳɼ״�����Ӧ����ʽΪ��CO2(g)��3H2(g) CH3OH(g)��H2O(g����H��0
CH3OH(g)��H2O(g����H��0
�ٹ�ҵ����������CO2��H2��ת����________���ǰ�ߴ������ߴ���һ�������жϡ�����Ϊ����״��IJ��ʿ��Բ�ȡ�Ĵ�ʩ��_______________�������㣩��
����һ���º����ܱ������г���1 mol CO2��3 mol H2����������Ӧ�����CO2��CH3OH(g)Ũ����ʱ��仯����ͼ��ʾ�����¶��µ�ƽ�ⳣ��Ϊ______��������λ��Ч���֣���
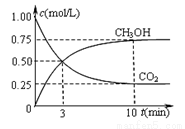
�ı��¶ȣ�ʹ��ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2��mol�� | H2��mol�� | CH3OH��mol�� | H2O��mol�� | |
��Ӧ�� ���º��� | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
��Ӧ�� ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�ٴﵽƽ��ʱ����Ӧ��Աȣ�ƽ�ⳣ��K(��)___K(��)�����������������=������ͬ����ƽ��ʱCH3OH��Ũ��c(��)___c(��)��
�ڶԷ�Ӧ��ǰ10 min�ڵ�ƽ����Ӧ���ʦ�(CH3OH)=______����30 minʱֻ���������ٳ���1 mol CO2(g)��1 mol H2O(g)����ƽ��_____�ƶ��������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com