
| 1.84g |
| 92g/mol |
| 1.44g |
| 18g/mol |
| 2.64g |
| 44g/mol |
| 92-3��12-8 |
| 16 |
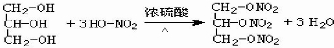 ��
��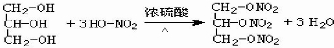 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ�Ž�һ��2011��2012ѧ��߶���ѧ�����п��Ի�ѧ���� ���ͣ�022
����������Ϣд������Ҫ����л���Ľṹ��ʽ��
(1)��ij�л���ķ�����Ϊ128�������л���Ϊ�����������ʽΪ________��������һ�ȴ���ֻ�ж��֣�������Ľṹ��ʽΪ________��������Ϊ�������������ʽΪ________��������Ľṹ��ʽ________�������л���Ϊ���ĺ�����������DZ���һԪ���л�������________��
(2)ij�л������C��H��O����Ԫ����ɣ���C��H��Oԭ�Ӹ���֮��Ϊ1��2��1�������л���Ϊȩ�࣬����ṹ��ʽΪ��________�������л���Ϊ���ᣬ����ṹ��ʽΪ________�������л���Ϊ���࣬����ṹ��ʽΪ________�������л���Ϊ���࣬����ṹ��ʽΪ________�������л������ʽΪC3H6O3���������Ӹ��л�����γ���Ԫ��״������C3H6O3�ṹ��ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
43.![]() ��
��![]() �ṹ�ϵIJ�������������������IJ�ͬ����14CO2��̼�ڸ��������·�����Ӧ��
�ṹ�ϵIJ�������������������IJ�ͬ����14CO2��̼�ڸ��������·�����Ӧ��
14CO2+C![]() 2CO���ﵽ��ѧƽ���ƽ�������к�14C�����У�������
2CO���ﵽ��ѧƽ���ƽ�������к�14C�����У�������
A.14CO2������������
B.14CO2��14CO
C.14CO2��14CO��14C
D.14CO
44.���п�����ַ���ֵ�����������14C�ⶨ������ǣ�������
A.ս��������Ĺ����ͭ���ӣ����Լ2400�꣩
B.����Ҥ�Ļ���ַ���ڣ����Լ5300�꣩
C.��ʼ�ʱ���ٸ�����Լ2200�꣩
D.Ԫı�˵��ųݣ����Լ170���꣩
45.������ͬλ�زⶨҲ������ѧ�о���һ�ֳ����ֶΡ���ѧ��������14C�Ժ�ķ����ַ�Ŵ浾�ȵ�������вⶨ�������н���C��H��O����Ԫ����ɵ��л�����֬��������������������ɶ����ǽ���14C�ⶨ��
������ͬλ�����Ŵ�ѧ�о���Ӧ�õ�һ����������������������ʵ�飬�ڸ�ʵ���У���ѧ�������˷����Ե�35S��32P���Ӷ��õ���������������ֱ��֤�ݡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com