

·ÖĪö £Ø1£©¢ŁīŃŹĒ22ŗÅŌŖĖŲ£¬Ti2+ŗĖĶāÓŠ20øöµē×Ó£¬øł¾Ż¹¹ŌģŌĄķŹéŠ“Ę仳Ģ¬ŗĖĶāµē×ÓÅŲ¼Ź½£»
¢Śøł¾Ż¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪČ·¶Øæռ乹ŠĶ£»
¢ŪĶ¬Ņ»ÖÜĘŚŌŖĖŲ£¬ŌŖĖŲµÄµēøŗŠŌĖę×ÅŌ×ÓŠņŹżµÄŌö“ó¶ųŌö“ó£¬ŌŖĖŲµÄµŚŅ»µēĄėÄÜĖę×ÅŌ×ÓŠņŹżµÄŌö“ó¶ų³ŹŌö“óµÄĒ÷ŹĘ£¬µ«µŚIIA×åŗĶµŚVA×åŌŖĖŲµŚŅ»µēĄėÄÜ“óÓŚĘäĻąĮŚŌŖĖŲ£¬Ō×ӵĹģµĄÖŠµē×Ó“¦ÓŚ°ėĀś”¢Č«Āś”¢Č«æÕŹ±±Č½ĻĪČ¶Ø£»
£Ø2£©a£®øł¾Ż¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪČ·¶ØŌӻƷ½Ź½£»
b£®Ķ¬Ņ»Ö÷×åŌŖĖŲµÄĒā»ÆĪļÖŠ£¬ŗ¬ÓŠĒā¼üµÄĒā»ÆĪļ·Šµć½Ļøߣ»
c£®Ģį¹©¹Āµē×Ó¶ŌµÄŌ×ÓŹĒÅäŌ×Ó£»
d£®CN-µÄ½į¹¹ŗĶµŖĘų·Ö×ÓĻąĖĘ£¬øł¾ŻµŖĘų·Ö×ӵĵē×ÓŹ½ÅŠ¶Ļ£»
£Ø3£©¢Łøł¾ŻĻąĖĘĻąČÜŌĄķČ·¶Ø·Ö×ӵļ«ŠŌ£»
¢ŚĄūÓĆ¾łĢƷؼĘĖć£»
£Ø4£©ĄūÓĆ¾łĢƷؼĘĖćøĆ¾§°ūÖŠĆ¾”¢ĒāŌ×ÓøöŹż£¬ŌŁøł¾ŻV=$\frac{m}{¦Ń}$½ųŠŠ¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©¢ŁīŃŹĒ22ŗÅŌŖĖŲ£¬Ti2+ŗĖĶāÓŠ20øöµē×Ó£¬øł¾Ż¹¹ŌģŌĄķÖŖĘ仳Ģ¬ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p63d2»ņ[Ar]3d2£¬¹Ź“š°øĪŖ£ŗ1s22s22p63s23p63d2»ņ[Ar]3d2£»
¢ŚBH4-ÖŠBŌ×Ó¼Ū²ćµē×Ó¶Ō=4+$\frac{1}{2}$£¬ĒŅƻӊ¹Āµē×Ó¶Ō£¬ĖłŅŌŹĒÕżĖÄĆęĢå½į¹¹£¬¹Ź“š°øĪŖ£ŗÕżĖÄĆęĢ壻
¢ŪCŗĶBŹōÓŚĶ¬Ņ»ÖÜĘŚĒŅCµÄŌ×ÓŠņŹż“óÓŚB£¬ĖłŅŌµēøŗŠŌC£¾B£¬NŌ×ÓÖŠ2p¹ģµĄ“¦ÓŚ°ė³äĀś×“Ģ¬£¬OŌ×ÓÖŠ2p¹ģµĄ¼Č²»ŹĒ°ė³äĀśŅ²²»ŹĒČ«æÕ»ņČ«Āś£¬ĖłŅŌNŌ×ÓµŚŅ»µēĄėÄܱČOŌ×Ó“ó£¬
¹Ź“š°øĪŖ£ŗ£¾£»£¾£»N µÄp¹ģµĄĪŖ°ė³äĀś×“Ģ¬£¬±ČOµÄדĢ¬øüĪČ¶Ø£»
£Ø2£©A£®NH3·Ö×ÓÖŠNŌ×Óŗ¬ÓŠ3øö¹²ÓƵē×Ó¶ŌŗĶŅ»øö¹Āµē×Ó¶Ō£¬ĖłŅŌĘä¼Ū²ćµē×Ó¶ŌŹĒ4£¬²ÉÓĆsp3Ōӻƣ¬¹ŹÕżČ·£»
B£®ĻąĶ¬Ń¹ĒæŹ±£¬°±ĘųÖŠŗ¬ÓŠĒā¼ü£¬PH3ÖŠ²»ŗ¬Ēā¼ü£¬ĖłŅŌNH3·Šµć±ČPH3øߣ¬¹ŹÕżČ·£»
C£®[Cu£ØNH3£©4]2+Ąė×ÓÖŠ£¬NŌ×ÓĢį¹©¹Āµē×Ó¶Ō£¬ĖłŅŌNŌ×ÓŹĒÅäĪ»Ō×Ó£¬¹ŹÕżČ·£»
D£®CN-µÄµē×ÓŹ½ĪŖ £¬¹Ź“ķĪó£»
£¬¹Ź“ķĪó£»
¹ŹŃ”ABC£»
£Ø3£©¢Ł±½”¢CS2¶¼ŹĒ·Ē¼«ŠŌ·Ö×Ó£¬øł¾ŻĻąĖĘĻąČÜŌĄķÖŖ£¬C60ŹĒ·Ē¼«ŠŌ·Ö×Ó£¬¹Ź“š°øĪŖ£ŗ·Ē¼«ŠŌ£»
¢ŚĄūÓĆ¾łĢÆ·ØÖŖ£¬ĆæøöĢ¼Ō×Óŗ¬ÓŠ$\frac{1}{2}$øö¦Ņ ¼ü£¬ĖłŅŌ1mol C60·Ö×ÓÖŠ£¬ŗ¬ÓŠ¦Ņ ¼üŹżÄæ=$\frac{3}{2}$”Į1mol”Į60”ĮNA/mol=90NA£¬¹Ź“š°øĪŖ£ŗ90NA£»
£Ø4£©øĆ¾§°ūÖŠĆ¾Ō×ÓøöŹż=$\frac{1}{8}$£¬Ō×ÓøöŹż=2+4”Į$\frac{1}{2}$£¬V=$\frac{m}{¦Ń}$=$\frac{\frac{M}{{N}_{A}}£Ø24”Į2+1”Į4£©}{a}$cm3=$\frac{52}{a{N}_{A}}$cm3£¬¹Ź“š°øĪŖ£ŗ$\frac{52}{a{N}_{A}}$£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹ½į¹¹ŗĶŠŌÖŹ£¬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢ŌӻƷ½Ź½µÄÅŠ¶Ļ”¢æռ乹ŠĶµÄÅŠ¶Ļ”¢¾§°ūµÄ¼ĘĖćµČÖŖŹ¶µć£¬ÄѵćŹĒ¾§°ūµÄ¼ĘĖć£¬Įé»īŌĖÓĆ¹«Ź½ŹĒ½ā±¾Ģā¹Ų¼ü£¬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ѧĻ°»ÆѧӦøĆĆ÷Č·”°“ÓÉś»īÖŠĄ“£¬µ½Éś»īÖŠČ„”±µÄµĄĄķ£®
ѧĻ°»ÆѧӦøĆĆ÷Č·”°“ÓÉś»īÖŠĄ“£¬µ½Éś»īÖŠČ„”±µÄµĄĄķ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2CO3”¢BaCl2 | B£® | HCl”¢KNO3 | C£® | HCl”¢Na2CO3 | D£® | Na2CO3”¢KNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 15 | B£® | 23 | C£® | 28 | D£® | 32 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2mol A+1mol B | |
| B£® | 1mol C+1mol D | |
| C£® | 2mol C+2mol D | |
| D£® | 0.5mol A+0.5mol B+0.5mol C+1mol D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | WO3ŗĶWO2.9ŹĒĶ¬·ÖŅģ¹¹Ģå | |
| B£® | ÓÉWO3ÖʱøWO2.9µÄ¹ż³Ģ·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦ | |
| C£® | 18g H2OŌŚWO2.9µÄøߊ§“ß»ÆĻĀ²śÉśH2Ģå»żĪŖ22.4L | |
| D£® | ĄūÓĆÕāÖÖŠĀŠĶ“߻ƼĮ·Ö½āĖ®µÄĶ¬Ź±æɷųöČČĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
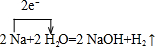 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¾Ę¾« | B£® | ¶žŃõ»ÆĮņ | C£® | Ņ»Ė®ŗĻ°± | D£® | ĮņĖį±µ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com