
����Ŀ�����
��1����ת����Һʱ�в�����Һ�����������Ƶ���ҺŨ��___________���ƫ�ߡ�����ƫ�͡����䡱����ͬ����
��2��ת����Һ��û����ˮϴ���ձ��ڱڣ���ҺŨ�Ƚ�__________��
��3����ˮ��������ƿ�Ŀ̶��ߣ���ҺŨ�Ƚ�__________����ʱӦ��_______________��
��4����ˮ����ʱ���ӿ̶��ߣ���ҺŨ�Ƚ�__________��
��5�����ݺ���Һҡ�ȣ�����Һ���½����ּ�ˮ���̶��ߣ���ҺŨ�Ƚ�_________��
��6���ռ��ܽ��û�лָ������£���ת�Ƶ�����ƿ�н������ƣ�Ũ�Ƚ�____________��
���𰸡�ƫ�� ƫ�� ƫ�� �������� ƫ�� ƫ�� ƫ��
��������
��1����ת����Һʱ�в�����Һ������������ʧ������c=![]() ��֪�������Ƶ���ҺŨ��ƫ�ͣ�
��֪�������Ƶ���ҺŨ��ƫ�ͣ�
��2��ת����Һ��û����ˮϴ���ձ��ڱڣ�������ʧ������c=![]() ��֪����ҺŨ�Ƚ�ƫ����
��֪����ҺŨ�Ƚ�ƫ����
��3����ˮ��������ƿ�Ŀ̶��ߣ�������Һ���ƫ����c=![]() ��֪����ҺŨ�Ƚ�ƫ�ͣ���ʱӦ������������
��֪����ҺŨ�Ƚ�ƫ�ͣ���ʱӦ������������
��4����ˮ����ʱ���ӿ̶��ߣ�������Һ���ƫ�٣�����c=![]() ��֪����ҺŨ�Ƚ�ƫ����
��֪����ҺŨ�Ƚ�ƫ����
��5�����ݺ���Һҡ�ȣ�����Һ���½����ּ�ˮ���̶��ߣ�����������ˮ������c=![]() ��֪����ҺŨ�Ƚ�ƫ�ͣ�
��֪����ҺŨ�Ƚ�ƫ�ͣ�
��6���ռ��ܽ��û�лָ������£���ת�Ƶ�����ƿ�н������ƣ��ռ�����ˮ���ȣ���������������ԭ����������Һ���ƫС������c=![]() ��֪��Ũ�Ƚ�ƫ����
��֪��Ũ�Ƚ�ƫ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������������Ҫ�����ã������ĺ����������� NaClO2��NaClO��ClO2��Cl2��
�ش��������⣺
��1����̬ Cl ԭ�Ӻ�����______�ֲ�ͬ�����ĵ��ӣ�Cl Ԫ�ش���Ԫ�����ڱ���______�������̬ԭ���У��������ռ�ݵĵ���������ͼΪ���ε��ܼ���_______����
��2��NaClO2 �����Ļ�ѧ��������___________��
��3��������Ͷ��ˮ�У�һ��ʱ����백��ϵõ�һ�ֽ�����Ȱ�(NH2C1)��NH2C1 �����Ի�����������Ҳ��һ��ǿɱ���������������ڳ�������ˮ��������������___________���û�ѧ����ʽ��ʾ����
��4������Ч�Ⱥ��������������������������������������䶨����ÿ�˺��������������������൱�ڶ��ٿ� Cl2 �������������������ֺ���������������������ǿ����_____��
��5������ˮ����ClO2�������ˮ�У�Ҫ��ClO2��Ũ����0.1��0.8 mg��L-1֮�䡣�õ�������� ClO2�����������ˮ��ClO2 Ũ�ȵ�ʵ�鲽�����£�
���� I��ȡһ�������ˮ�������������⻯�أ���������������Һ�������ԣ������������Һ��
������� Na2S2O3 ��Һ�ζ����� I �����õ���Һ��
��֪��a������ʱ����ͬ pH �����������������±���ʾ��
pH | 2 | 7 |
�������� | Cl- | ClO2 |
b. 2S2O32-+I2 =S4O62-+2I ��
�� ������з�Ӧ����ʱ��Һ��____�����ɫ������ɫ������
�� ��ˮ�������Ϊ1.0L���ڲ����������10mL1.0��10-3mol��L-lNa2S2O3��Һ�����ˮ����c(ClO2)=____mg��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯ�Ͳ�ƷA(C3H6)Ϊ��Ҫԭ�ϣ��ϳɾ��й㷺��;���л�����PMMA����������(���ֲ��������ʡ��)
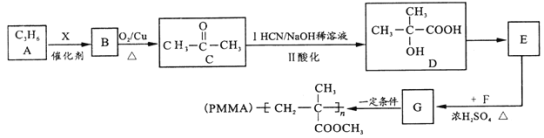
�Իش��������⣺
��1��B������________�� D ���������_________��
��2��E��G �Ļ�ѧ����ʽΪ____����Ӧ������_________��
��3��T��G��ͬ���칹����1molT����������Cu(OH)2����Һ��Ӧ�������2molCu2O������T�Ľṹ��________��(��������ṹ)��
��4�����߷��ӻ�����PMMA ����Է�������Ϊ1.5��106����ۺ϶�Ϊ_______��
��5�������������̣���![]() Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ� (����ԭ����ѡ)����ƺϳ�·�ߣ�________________________��
(����ԭ����ѡ)����ƺϳ�·�ߣ�________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ں��ա����ܡ���صȸ����������Ҫ���á���ﮱ���Ϊ��21���͵���Դ�����������������գ�
(1)�λ��Ԫ�����ڱ���________��﮵ĺܶѧ������þ���ƣ�������Ԫ�������ɽ��н��ͣ�_________��
(2)�⻯ﮣ�LiH�������ӻ����д���������ӵĵ���ʽ_______________��LiH�������Ӱ뾶���������Ӱ뾶����ԭ����__________________________________________________��
(3) ��ҵ����Li2CO3��ȡ﮵Ļ����P����ﮡ�̼ԭ�Ӻ��������________�ֲ�ͬ�����ĵ��ӣ��������������ӵ����������������ȣ����������������Ĺ����_____________________��
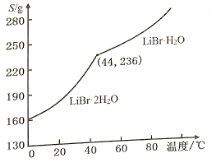
(4)��Li2CO3����ˮ��Һ���Ʊ�LiBr��H2O���������£�

�ٺϳ�ʱ��������LiBr�⣬�����������ֲ������ѭ�������壬��ȫ���ﲢ��ƽ�÷�Ӧ�Ļ�ѧ����ʽ��
Li2CO3+Br2+ NH3��H2O��LiBr+��___�� __________��
���廯﮵��ܽ�����¶ȱ仯������ͼ��ʾ���벹ȫ����Һ�еõ�LiBr��H2O�����ʵ�鲽�裺_____�����ˡ����Ҵ�ϴ�ӣ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ҫ��ش��������⡣
��1����ͬ������SO2�����SO3���壬�������֮��Ϊ_____����ԭ����֮��Ϊ_____����ͬ�����£�ͬ��ͬѹ�������֮��Ϊ_____���ܶ�֮��Ϊ_____��
��2��SO2��O2�Ļ�������У���Ԫ�ص���������Ϊ70%����SO2��O2�����ʵ���֮����_____�����ֻ��������ܶ���ͬ��ͬѹ�������ܶȵ�_____����
��3����2.4 mol��L��1��H2SO4��Һ����100 mLŨ��Ϊ0.2 mol��L��1��ϡ���ᣬ����ȡ2.4 mol��L��1��H2SO4��Һ�������_____mL��
��4���Է������в�����������Һ��Ũ���к�Ӱ�죨����ƫ������ƫ����������Ӱ��������
��δϴ���ձ��Ͳ�������ʹ������ҺŨ��_____��
�ڶ���ʱ���ӿ̶��ߣ�ʹ������ҺŨ��_____��
��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ��ʹ������ҺŨ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������H1N1���в�������ȫ��㷺�����������ཡ������ᾭ�ô����˾�ĸ���Ӱ�졣�ҹ���ȡ����Ӧ�Դ�ʩ��ʹ�����õ�����Ч�Ŀ��ƣ��Ӻܴ�̶��ϼ�������ʧ�����������Һ������û��
��1����������Һ�ǽ�����ͨ��NaOH��Һ�У����������ӷ�Ӧ����ʽΪ_____��
��2��ȡ��������Һ�μ�AgNO3��Һ�������dz��ְ�ɫ������˵������Һ����___���ӡ�
��3������Һϡ�ͺ������ڿ����У�����������Ư���Ե����ʣ�д���˹��̵����ӷ�Ӧ����ʽ��____����������Ư��������Ϊ����ǿ�����ԣ�������Ҳ�ܲ��ȶ���д�����ֽ�Ļ�ѧ����ʽ�� ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ļ��������[(CH3)4NOH]��ǿ������¼װ�(CH3NH2��H2O)�ĵ��볣��ΪKb����pKb=��lgKb=3.38�������£��������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol��L��1���ļ����������Һ�ͼװ���Һ���ֱ�μ�Ũ��Ϊ0.1mol��L��1�����ᣬ��Һ�ĵ���������������Ĺ�ϵ��ͼ��ʾ��
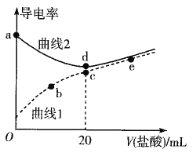
����˵����ȷ����
A. ����1�����ļ����������Һ
B. ��b��c��e�����У�ˮ�ĵ���̶����ĵ���e
C. b����Һ�д���c(H+)=c(OH��)+c(CH3NH2��H2O)
D. �����£�CH3NH3Clˮ�ⳣ����������Ϊ10��11
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��NaOH��Ħ������Ϊ��__________ ��
��2��24.5��H2SO4�����ʵ���Ϊ��___________��
��3������£�8.96LCO2������Ϊ��___________��
��4����3.01��1023��Nԭ�ӵ�NH3�ڱ���µ����Ϊ:_____________ ��
��5��3g����п��������ϡ���ᷴӦ�����ɱ���µ��������Ϊ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com