
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2010?���գ��ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϣ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�
��2010?���գ��ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϣ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�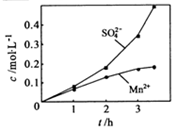
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϣ�
��ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϣ�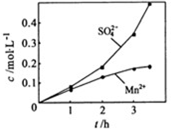
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�߶���ѧ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
�ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�
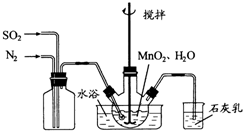
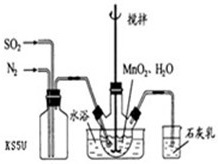
��1���Ʊ�MnSO4��Һ������ƿ�У�װ�ü�ͼ������һ����MnO2 ��ˮ�����裬ͨ�� SO2��N2������壬��Ӧ3h��ֹͣͨ��SO2��������ӦƬ�̣����ˣ���֪MnO2+H2SO3=MnSO4+H2O����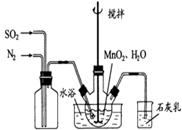
��ʯ�������ã� ��
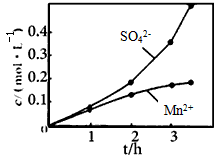
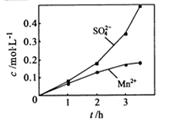
����ʵ���н� ���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ���� ��
���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ���� ��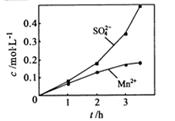
��2���Ʊ��ߴ�MnCO3���壺��֪MnCO3������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻Mn(OH)2��ʼ����ʱpH="7.7," �벹���ɣ�1���Ƶõ�MnSO4��Һ�Ʊ��ߴ�MnCO3�IJ������裺
[ʵ���п�ѡ�õ��Լ���Ca(OH)2��NaHCO3��Na2CO3��C2H5OH]��
�� ��
�� ��
�ۼ���SO42-�Ƿ�ϴ�ӳ�ȥ�� ���������Ҵ�ϴ�ӣ� �ݵ���100����
��п���Ͻ����Ҫ�ɷ���Zn��Al��Cu��Si��Ԫ�ء�ʵ���Ҳⶨ����Cu�����IJ������£��ٳ�ȡ�úϽ���Ʒ1.1g����HCl��H2O2�ܽ����г�ȥ����H2O2�����ˣ���Һ������250mL����ƿ�С�
������Һ����ȡ50.00mL��Һ��250mL����ƿ�У�������Һ��pH=3��4���������KI��Һ������CuI��I2����ָʾ������0.01100mol��L-1Na2S2O3��Һ�ζ����ɵ�I2���յ㣨��Ӧ��I2 + 2S2O32- = 2I- + S4O62-��������Na2S2O3��Һ6.45mL��
��1���жϲ���ڵζ��յ�ķ����� ��
��2������Ͻ���Cu���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
�ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�
��1���Ʊ�MnSO4��Һ������ƿ�У�װ�ü�ͼ������һ����MnO2 ��ˮ�����裬ͨ�� SO2��N2������壬��Ӧ3h��ֹͣͨ��SO2��������ӦƬ�̣����ˣ���֪MnO2+H2SO3=MnSO4+H2O����
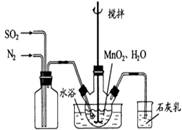
��ʯ�������ã� ��
����ʵ���н� ���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ����
��
���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ����
��
��2���Ʊ��ߴ�MnCO3���壺��֪MnCO3������ˮ���Ҵ�����ʪ ʱ�ױ�����������100�濪ʼ�ֽ⣻Mn(OH)2��ʼ����ʱpH=7.7, �벹���ɣ�1���Ƶõ�MnSO4��Һ�Ʊ��ߴ�MnCO3�IJ������裺
[ʵ���п�ѡ�õ��Լ���Ca(OH)2��NaHCO3��Na2CO3��C2H5OH]��
�� ��
�� ��
�ۼ���SO42-�Ƿ�ϴ�ӳ�ȥ�� ���������Ҵ�ϴ�ӣ� �ݵ���100����
��п���Ͻ����Ҫ�ɷ���Zn��Al��Cu��Si��Ԫ�ء�ʵ���Ҳⶨ����Cu�����IJ������£��ٳ�ȡ�úϽ���Ʒ1.1g����HCl��H2O2�ܽ����г�ȥ����H2O2�����ˣ���Һ������250mL����ƿ�С�
������Һ����ȡ50.00mL��Һ��250mL����ƿ�У�������Һ��pH=3��4���������KI��Һ������CuI��I2����ָʾ������0.01100mol��L-1Na2S2O3��Һ�ζ����ɵ�I2���յ㣨��Ӧ��I2 + 2S2O32- = 2I- + S4O62-��������Na2S2O3��Һ6.45mL��
��1���жϲ���ڵζ��յ�ķ����� ��
��2������Ͻ���Cu���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϣ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�
�ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϣ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com