
½«4 mol AŗĶ2 mol B·ÅČė2 LĆܱÕČŻĘ÷ÖŠ·¢Éś·“Ó¦£ŗ2A(g)£«B(g)  2C(g)””¦¤H<0”£4 sŗó·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±²āµĆCµÄÅضČĪŖ0.6 mol·L£1”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)”£
2C(g)””¦¤H<0”£4 sŗó·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±²āµĆCµÄÅضČĪŖ0.6 mol·L£1”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ (””””)”£
A£®4 sÄŚ£¬v(B)£½0.075 mol·L£1·s£1
B£®4 sŗóĘ½ŗāדĢ¬ĻĀ£¬c(A)”Ćc(C)£½2”Ć1
C£®“ļµ½Ę½ŗāדĢ¬ŗó£¬ČōֻɿøßĪĀ¶Č£¬ŌņCµÄĪļÖŹµÄĮæÅضČŌö“ó
D£®“ļµ½Ę½ŗāדĢ¬ŗó£¬ČōĪĀ¶Č²»±ä£¬ĖõŠ”ČŻĘ÷µÄĢå»ż£¬ŌņAµÄ×Ŗ»ÆĀŹ½µµĶ
 °Ł·Öѧɜ×÷Ņµ±¾ĢāĮ·ĶõĻµĮŠ“š°ø
°Ł·Öѧɜ×÷Ņµ±¾ĢāĮ·ĶõĻµĮŠ“š°ø »„¶ÆæĪĢĆĻµĮŠ“š°ø
»„¶ÆæĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷±ķŹöÓėŹ¾ŅāĶ¼Ķ¼ÖŠŅ»ÖĀµÄŹĒ
A£®Ķ¼¢Ł±ķŹ¾Ļņŗ¬M g2£«”¢Al3£«”¢NH4£«µÄŃĪČÜŅŗÖŠ
g2£«”¢Al3£«”¢NH4£«µÄŃĪČÜŅŗÖŠ µĪ¼ÓNaOHČÜŅŗŹ±£¬³ĮµķµÄĮæÓėNaOHµÄĢå»żµÄ¹ŲĻµĶ¼”£ŌņČżÖÖĄė×ÓµÄĪļÖŹµÄĮæÖ®±ČĪŖ£ŗn(Mg2£«)”Ćn(Al3£«):n( NH4£«)£½2”Ć3”Ć2
µĪ¼ÓNaOHČÜŅŗŹ±£¬³ĮµķµÄĮæÓėNaOHµÄĢå»żµÄ¹ŲĻµĶ¼”£ŌņČżÖÖĄė×ÓµÄĪļÖŹµÄĮæÖ®±ČĪŖ£ŗn(Mg2£«)”Ćn(Al3£«):n( NH4£«)£½2”Ć3”Ć2
B£®Ķ¼¢ŁÖŠŹ¹ÓƵÄNaOHµÄÅضČĪŖ10 mol/L
C£®Ķ¼¢Ś±ķŹ¾25”ꏱ£¬ ÓĆ0.1 mol”¤L£1ŃĪĖįµĪ¶Ø20 mL 0.1 mol”¤L£1 NaOHČÜŅŗ£¬ČÜŅŗµÄpHĖę¼ÓČėĖįĢå»żµÄ±ä»Æ
ÓĆ0.1 mol”¤L£1ŃĪĖįµĪ¶Ø20 mL 0.1 mol”¤L£1 NaOHČÜŅŗ£¬ČÜŅŗµÄpHĖę¼ÓČėĖįĢå»żµÄ±ä»Æ
D£®Ķ¼¢ŪÖŠĒśĻß±ķŹ¾3N2(g)+N2(g) 2NH3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»Æ£¬ČōŹ¹ÓĆ“ß»Æ¼Į£¬Bµć»įÉżøß
2NH3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»Æ£¬ČōŹ¹ÓĆ“ß»Æ¼Į£¬Bµć»įÉżøß
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹ūÖŌŚÉś²ś¹ż³ĢÖŠĢķ¼ÓŃĒĮņĖį¼°ĘäŃĪĄą£¬ŅŌŅÖÖĘÓŠŗ¦Ī¢ÉśĪļµÄÉś³¤¼°æ¹Ńõ»Æ”£Ä³»ÆѧŠĖȤŠ”×é²éŌÄĪÄĻ×ŗó£¬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶Øij¹ūÖѳʷ֊SO2²ŠĮōĮ攣
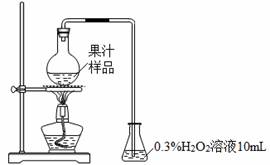
ŹµŃé²½Öč£ŗ
£Ø¢”£©ŌŚÉÕĘæÖŠ¼ÓČė50.00 mL¹ūÖѳʷÓė3mLÅØĮņĖįµÄ»ģŗĻŅŗ£¬¼ÓČČ10 min”£
£Ø¢¢£©ŅĘ³ö׶ŠĪĘ棬ŃøĖŁµĪČė2~3µĪ·ÓĢŖŹŌŅŗ£¬ÓĆ0.01000 mol”¤L£1NaOH±ź×¼ČÜŅŗµĪ¶Ø”£
»Ų“šĪŹĢā£ŗ
£Ø1£©ŹµŃéŹŅÅäÖĘNaOH±ź×¼ČÜŅŗŹ±£¬²»ŠčŅŖÓƵ½µÄŅĒĘ÷ŹĒ ”£
A£®ČŻĮæĘæ B£®½ŗĶ·µĪ¹Ü C£®ÉÕĘæ D£®²£Į§°ō
£Ø2£©½«50. 00 mL¹ūÖѳʷÓė3mLÅØĮņĖį»ģŗĻµÄ²Ł×÷ŹĒ ”£
£Ø3£©×¶ŠĪĘæÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø4£©Čō²½Öč£Ø¢¢£©ĻūŗÄNaOH±ź×¼ČÜŅŗ25. 00 mL£¬Ōņ¹ūÖѳʷ֊SO2²ŠĮōĮæŹĒ g/L”£
£Ø5£©ČōøÄÓĆ0.5%µÄµāĖ®10 mL×÷ĪüŹÕŅŗ£¬ŹµŃéÖŠĻūŗÄNaOH±ź×¼ČÜŅŗĢå»ż ”£
A£®V = 25 mL B£®25 mL£¼V£¼50 mL C£®V£¾50 mL
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚČŻ»żĪŖ1.00 LµÄČŻĘ÷ÖŠ£¬ĶØČėŅ»¶ØĮæµÄN2O4£¬·¢Éś·“Ó¦N2O4(g)  2NO2(g)£¬ĖęĪĀ¶ČÉżøߣ¬»ģŗĻĘųĢåµÄŃÕÉ«±äÉī”£
2NO2(g)£¬ĖęĪĀ¶ČÉżøߣ¬»ģŗĻĘųĢåµÄŃÕÉ«±äÉī”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)·“Ó¦µÄ¦¤H________0(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)£»100 ”ꏱ£¬ĢåĻµÖŠø÷ĪļÖŹÅضČĖꏱ¼ä±ä»ÆČēĶ¼ĖłŹ¾”£ŌŚ0”«60 sŹ±¶Ī£¬·“Ó¦ĖŁĀŹv(N2O4)ĪŖ________mol·L£1·s£1£»·“Ó¦µÄĘ½ŗā³£ŹżK1ĪŖ________”£
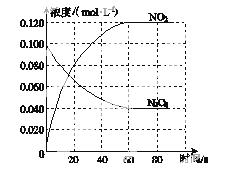
(2)100 ”ꏱ“ļĘ½ŗāŗó£¬øı䷓ӦĪĀ¶ČĪŖT£¬c(N2O4 )ŅŌ0.002 0 mol·L£1·s£1µÄĘ½¾łĖŁĀŹ½µµĶ£¬¾10 sÓÖ“ļµ½Ę½ŗā”£
)ŅŌ0.002 0 mol·L£1·s£1µÄĘ½¾łĖŁĀŹ½µµĶ£¬¾10 sÓÖ“ļµ½Ę½ŗā”£
¢ŁT________100 ”ę(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)£¬ÅŠ¶ĻĄķÓÉŹĒ____________________________”£
¢ŚĮŠŹ½¼ĘĖćĪĀ¶ČTŹ±·“Ó¦µÄĘ½ŗā³£ŹżK2£ŗ_______________________________________
________________________________________________________________________ӣ
(3)ĪĀ¶ČTŹ±·“Ó¦“ļĘ½ŗāŗ󣬽«·“ӦȯĘ÷µÄČŻ»ż¼õÉŁŅ»°ė£¬Ę½ŗāĻņ________(Ģī”°Õż·“Ó¦”±»ņ”°Äę·“Ó¦”±)·½ĻņŅĘ¶Æ£¬ÅŠ¶ĻĄķÓÉŹĒ__________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖij»Æѧ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK£½ £¬ŌŚ²»Ķ¬µÄĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżČēĻĀ±ķ£ŗ
£¬ŌŚ²»Ķ¬µÄĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżČēĻĀ±ķ£ŗ
| t ”ę | 700 | 800 | 830 | 1 000 | 1 200 |
| K | 1.67 | 1.11 | 1.00 | 0.60 | 0.38 |
ĻĀĮŠÓŠ¹ŲŠšŹö²»ÕżČ·µÄŹĒ (””””)”£
A£®øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒCO(g)£«H2O(g)  CO2(g)£«H2(g)
CO2(g)£«H2(g)
B£®ÉĻŹö·“Ó¦µÄÕż·“Ó¦ŹĒ·ÅČČ·“Ó¦
C£®ČōŌŚ1 LµÄĆܱÕČŻĘ÷ÖŠĶØČėCO2ŗĶH2ø÷1 mol,5 minŗóĪĀ¶ČÉżøßµ½830 ”ę£¬“ĖŹ±²āµĆCO2ĪŖ0.4 mol£¬øĆ·“Ó¦“ļµ½Ę½ŗāדĢ¬
D£®ČōĘ½ŗāÅØ¶Č·ūŗĻ¹ŲĻµŹ½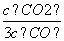 £½
£½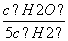 £¬Ōņ“ĖŹ±µÄĪĀ¶ČĪŖ1 000 ”ę
£¬Ōņ“ĖŹ±µÄĪĀ¶ČĪŖ1 000 ”ę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ļņ¼×ŅŅĮ½øöČŻ»ż¾łĪŖ1 LµÄŗćČŻČŻĘ÷ÖŠ£¬·Ö±š³äČė2 mol A”¢2 mol BŗĶ1 mol A”¢1 mol B”£ĻąĶ¬Ģõ¼žĻĀ(ĪĀ¶ČT ”ę)£¬·¢ÉśĻĀĮŠ·“Ó¦£ŗA(g)£«B(g)xC(g)””¦¤H<0”£²āµĆĮ½ČŻĘ÷ÖŠc(A)Ėꏱ¼ätµÄ±ä»ÆČēĶ¼ĖłŹ¾£ŗ
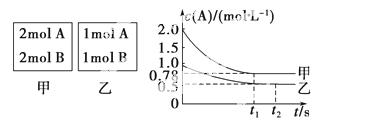
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŅŅČŻĘ÷ÖŠ£¬Ę½ŗāŗóĪļÖŹBµÄ×Ŗ»ÆĀŹĪŖ________”£
(2)x£½________”£
(3)T ”ꏱøĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ________”£
(4)ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)”£
A£®ĻņĘ½ŗāŗóµÄŅŅČŻĘ÷ÖŠ³äČėŗ¤ĘųæÉŹ¹c(A)Ōö“ó
B£®½«ŅŅČŻĘ÷µ„¶ĄÉżĪĀæÉŹ¹ŅŅČŻĘ÷ÄŚø÷ĪļÖŹµÄĢå»ż·ÖŹżÓė¼×ČŻĘ÷ÄŚĻąĶ¬
C£®ČōĻņ¼×ČŻĘ÷ÖŠŌŁ³äČė2 mol A”¢2 mol B£¬ŌņĘ½ŗāŹ±¼×ČŻĘ÷ÖŠ0.78 mol·L£1<c(A)<1.56 mol·L£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
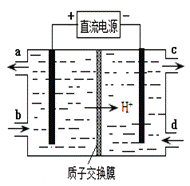
SO2”¢NOŹĒ“óĘųĪŪČ¾Īļ”£ĪüŹÕSO2 ŗĶNO£¬»ńµĆNa2S2O4ŗĶNH4NO3²śĘ·µÄĮ÷³ĢĶ¼ČēĻĀ£ØCeĪŖīęŌŖĖŲ£©£ŗ
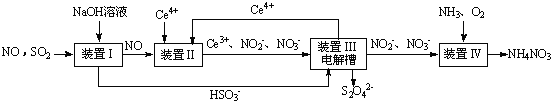
£Ø1£©×°ÖĆ¢ńÖŠÉś³ÉHSO3£µÄĄė×Ó·½³ĢĪŖ ”£
£Ø2£©ŗ¬Įņø÷Ī¢Į££ØH2SO3”¢HSO3£ŗĶSO32££©“ęŌŚÓŚSO2ÓėNaOHČÜŅŗ·“Ó¦ŗóµÄČÜŅŗÖŠ£¬ĖüĆĒµÄĪļÖŹµÄĮæ·ÖŹżX(i)ÓėČÜŅŗpH µÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£
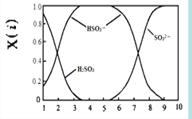
¢ŁĻĀĮŠĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄøŠņŗÅ£©”£
a£®pH=8Ź±£¬ČÜŅŗÖŠc(HSO3£) < c(SO32£)
b£®pH=7Ź±£¬ČÜŅŗÖŠc(Na+) =c(HSO3£)+c(SO32£)
c£®ĪŖ»ńµĆ¾”æÉÄÜ“æµÄNaHSO3£¬æɽ«ČÜŅŗµÄpHæŲÖĘŌŚ4”«5×óÓŅ
¢ŚĻņpH=5µÄNaHSO3ČÜŅŗÖŠµĪ¼ÓŅ»¶ØÅØ¶ČµÄCaCl2ČÜŅŗ£¬ČÜŅŗÖŠ³öĻÖ»ė×Ē£¬pH½µĪŖ2£¬ÓĆ»ÆŃ§Ę½ŗāŅʶÆŌĄķ½āŹĶČÜŅŗpH½µµĶµÄŌŅņ£ŗ ”£
£Ø3£©×°ÖĆ¢ņÖŠ£¬ĖįŠŌĢõ¼žĻĀ£¬NO±»Ce4+Ńõ»ÆµÄ²śĪļÖ÷ŅŖŹĒNO3£”¢NO2££¬Š“³öÉś³ÉNO3£µÄĄė×Ó·½³ĢŹ½ ”£
£Ø4£©×°ÖĆ¢óµÄ×÷ÓĆÖ®Ņ»ŹĒŌŁÉśCe4+£¬ĘäŌĄķČēĻĀĶ¼ĖłŹ¾”£
¢ŁÉś³ÉCe4+µÄµē¼«·“Ó¦Ź½ĪŖ ”£
¢ŚÉś³ÉCe4+“Óµē½ā²ŪµÄ £ØĢī×ÖÄøŠņŗÅ£©æŚĮ÷³ö”£
£Ø5£©ŅŃÖŖ½ųČė×°ÖĆ¢ōµÄČÜŅŗÖŠ£¬NO2£µÄÅضČĪŖa g·L-1£¬ŅŖŹ¹1 m3øĆČÜŅŗÖŠµÄNO2£ĶźČ«×Ŗ»ÆĪŖNH4NO3£¬ŠčÖĮÉŁĻņ×°ÖĆ¢ōÖŠĶØČė±ź×¼×“æöĻĀµÄO2 L”££ØÓĆŗ¬a“śŹżŹ½±ķŹ¾£¬¼ĘĖć½į¹ū±£ĮōÕūŹż£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Äų¾ßÓŠÓÅĮ¼µÄĪļĄķŗĶ»ÆѧĢŲŠŌ£¬ŹĒŠķ¶ąĮģÓņÓČĘäŹĒøß¼¼Źõ²śŅµµÄÖŲŅŖŌĮĻ”£ōŹ»ł·ØĢį“æ“ÖÄųÉę¼°µÄĮ½²½·“Ó¦ŅĄ“ĪĪŖ£ŗ
¢ŁNi(s)£«4CO(g)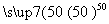 Ni(CO)4(g)£«Q
Ni(CO)4(g)£«Q
¢ŚNi(CO)4(g)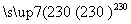 Ni(s)£«4CO(g)
Ni(s)£«4CO(g)
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
(1)ŌŚĪĀ¶Č²»±äµÄĒéæöĻĀ£¬ŅŖĢįøß·“Ó¦¢ŁÖŠNi(CO)4µÄ²śĀŹ£¬æɲÉČ”µÄ“ėŹ©ÓŠ____________”¢____________ ”£
(2)Čō·“Ó¦¢Ś“ļµ½Ę½ŗāŗ󣬱£³ÖĘäĖūĢõ¼ž²»±ä£¬½µµĶĪĀ¶Č£¬ÖŲŠĀ“ļµ½Ę½ŗāŹ±_______________ ”£
a£®Ę½ŗā³£ŹżKŌö“ó”””””””” b£®COµÄÅØ¶Č¼õŠ”
c£®NiµÄÖŹĮæ¼õŠ” d£®vÄę[Ni(CO)4]Ōö“ó
(3)¼ņŹöōŹ»ł·ØĢį“æ“ÖÄųµÄ²Ł×÷¹ż³Ģ”£
______________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅŠčÓĆ240mL 1mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ”£ĢīæÕ²¢Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1) ÅäÖĘĖłŠčĢå»żµÄ 1mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ
| Ó¦³ĘČ”ĒāŃõ»ÆÄĘ¹ĢĢåµÄÖŹĮæ/g | ӦєÓĆČŻĮæĘæµÄ¹ęøń/mL | ³żÉÕ±”¢ĶŠÅĢĢģĘ½”¢ČŻĮæĘ攢ĮæĶ²Ķā»¹ŠčµÄĘäĖüŅĒĘ÷ |
(2)ÅäÖĘŹ±£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ(×ÖÄø±ķŹ¾£¬Ćæøö×ÖÄøÖ»ÄÜÓĆŅ»“Ī) £»
A£®ÓĆ30mLĖ®Ļ“µÓÉÕ±2”Ŗ3“Ī£¬Ļ“µÓŅŗ¾ł×¢ČėČŻĮæĘ棬Õńµ“
B£®ÓĆĶŠÅĢĢģĘ½×¼Č·³ĘČ”ĖłŠčµÄĒāŃõ»ÆÄĘ¹ĢĢ壬µ¹ČėÉÕ±ÖŠ£¬ŌŁ¼ÓČėÉŁĮæĖ®£ØŌ¼30mL£©£¬ÓĆ²£Į§°ōĀżĀż½Į¶Æ£¬Ź¹Ęä³ä·ÖČܽā
C£®½«ŅŃĄäČ“µÄČÜŅŗŃŲ²£Į§°ō×¢ČėŅ»¶Ø¹ęøńµÄČŻĮæĘæÖŠ
D£®½«ČŻĮæĘæøĒ½ō£¬Õńµ“£¬Ņ”ŌČ
E£®øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻąĒŠ
F£®¼ĢŠųĶłČŻĮæĘæÄŚŠ”ŠÄ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶Č1”Ŗ2cm“¦
(3)²Ł×÷AÖŠ£¬½«Ļ“µÓŅŗ¶¼ŅĘČėČŻĮæĘ棬ĘäÄæµÄŹĒ £¬ČÜŅŗ×¢ČėČŻĮæĘæĒ°Šč»Öø“µ½ŹŅĪĀ£¬ÕāŹĒŅņĪŖ
£»
(4)Čō³öĻÖČēĻĀĒéæö£¬¶ŌĖłÅäČÜŅŗÅØ¶Č½«ÓŠŗĪÓ°Ļģ£ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©?
Čōƻӊ½ųŠŠA²Ł×÷ £»Čō¼ÓÕōĮóĖ®Ź±²»É÷³¬¹żĮĖæĢ¶Č £»Čō¶ØČŻŹ±ø©ŹÓæĢ¶ČĻß___________________”£
(5)ČōŹµŃé¹ż³ĢÖŠ³öĻÖČēĻĀĒéæöČēŗĪ“¦Ąķ£æ
¼ÓÕōĮóĖ®Ź±²»É÷³¬¹żĮĖæĢ¶Č £»
ĻņČŻĮæĘæÖŠ×ŖŅĘČÜŅŗŹ±(ŹµŃé²½Öč¢Ś)²»É÷ÓŠŅŗµĪµōŌŚČŻĮæĘæĶāĆę
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com