
����������й�������Һ���ʱ�ı仯�����
(1)��ʯī���缫������ͼװ�õ��AlCl3��Һ���������������ݣ��������г������ɡ�������⣬��������������Һ�л��ɹ۲쵽�������� �����ʹ���������ӷ���ʽ�� ��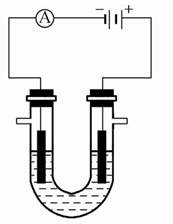
(2)����ʯī���缫���NaCl��Al2(SO4)3�Ļ����Һ�������Һ�ж��ߵ����ʵ���Ũ�ȷֱ�Ϊ3 mol��L-1��0.5 mol��L-1�������б�ʾ�����̵�������ȷ���� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ѡ�����˵IJ��Ϻ��Լ����һ��ԭ���,������з�Ӧ:
Zn+CuSO4=ZnSO4+Cu
(1)����װ��ͼ,�����������ֲ������ơ�
(2)����������������,����������������,�������Һ������������
(3)д���缫��Ӧʽ:
����: _______________________
����: _______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�����ͨѶ����ͨ���ճ����������Ź㷺��Ӧ�á�
��1��Ŀǰ���õ����ӣ�Ni��Cd����أ������ܷ�Ӧ���Ա�ʾΪ��
Cd��2NiO��OH����2H2O 2Ni��OH��2��Cd��OH��2
2Ni��OH��2��Cd��OH��2
��֪Ni��OH��2��Cd��OH��2��������ˮ���������ᣬ����˵����ȷ����________������ĸ��ţ���
�����Ϸ�Ӧ�ǿ��淴Ӧ�������Ϸ�Ӧ���ǿ��淴Ӧ���۳��ʱ��ѧ��ת��Ϊ���ܡ��ܷŵ�ʱ��ѧ��ת��Ϊ����
| A���٢� | B���ڢ� |
| C���٢� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)�����������������õ�ⷨ�ɻ���WC��Co���������̼�ͼ���£�
��1�����ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦʽΪ________________________________________________________________________��
��2���������������˱�����Ҫ�ɷ���________�����յ�ϴ��Һ����ˮ���Ƶ��Һ��Ŀ���ǻ����������е�________��
��3����Һ�����Ҫ�ɷ���________��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ����____________________________________________��
��4����Co2O3��ԭ��Co�۵Ļ�ѧ��Ӧ����ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���������ʵ�����KI��CuCl2����ˮ���ö��Ե缫��⣬�õ�ⷴӦ�ɷ�Ϊ________���Σ�����һ���������������������������ӣ���
| �� | �൱�ڵ��ʲô��Һ | ���ӷ���ʽ |
| �� | | |
| �� | | |
| �� | | |
| �� | | |
| �� | | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ⷨ�ڽ�������������������������ˮ������ʮ����Ҫ�����á�
��1����ͼΪ��⾫������ʾ��ͼ��________����a��b����Ϊ�������ʵĴ�������b������������ɫ�������ɣ������ɸ�����ĵ缫��ӦʽΪ________________________��
AgNO3��HNO3��Һ
��2����ⷨ�������Ժ�����ˮ����Ҫ����Cr2O72-��ʱ�����������������������������д��ڷ�ӦCr2O72-��6Fe2����14H��=2Cr3����6Fe3����7H2O�����Cr3����Cr��OH��3��ʽ��ȥ���ش��������⣺
��д���缫��Ӧʽ������________________������________________��
�ڵ�����1 mol Cr��OH��3ʱ����·��ת�Ƶ��ӵ����ʵ�������Ϊ________mol��
�۵���������Fe��OH��3�������ɣ�ԭ����_________________________________________________________________
________________________________________________________________��
��3����⽵�ⷨ����������ˮ�������ε���Ⱦ����⽵��NO3-��ԭ����ͼ��ʾ��
�ٵ�Դ����Ϊ________����A��B����������ӦʽΪ________________________________________________________________��
������������ת����2 mol���ӣ���Ĥ������Һ�������仯���m������m����Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�������֮����ת�������������ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ���أ���ͼ9��8����ԭ��ʾ��ͼ����Ӧԭ��Ϊ2Na��FeCl2 Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
���ʱ��____________��д�������ƣ��缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ________��
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ������ԭ��ʾ��ͼ��ͼ��ʾ��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98 g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
��Y�缫������________������________�����������ԭ������Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫��Ӧʽ��_______________________________________________________________________��
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҵ�ʵ��װ����ͼ��ʾ���������ֱ����ȼҵ����ʾ��ͼ���Ʊ������ѵ�ʾ��ͼ��
��ش��������⣺
(1)д����װ����̼������ĵ缫��Ӧʽ��_______________________________��
(2)��֪��5Cl2��I2��6H2O=10HCl��2HIO3������ʪ��ĵ���KI��ֽ������װ���е�̼������������Ϊ________________________________������װ����ת��0.02 mol���Ӻ�ֹͣʵ�飬�ձ�����Һ�����Ϊ200 mL�����ʱ��Һ��pH��________��(���������£��Ҳ����ǵ���������Ӧ)
(3)��ҵ�Ͼ����õ����ӽ���Ĥ�����ӽ���Ĥ�������ӽ���Ĥ�������ӽ���Ĥ���֣������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ��������װ���еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ�������ͼ��ʾ��װ�ã�Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�C������D������E����F��)��________(��ܡ����ܡ�)�������ӽ���Ĥ���������ӽ���Ĥ��
(4)�о����֣�������ʯī������������������������CaO������ʣ����ö�װ�û�ý����ƣ����Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
��д�������ĵ缫��Ӧʽ��___________________________________________��
�����Ʊ�������ǰ��CaO���������䣬��ԭ����(���ϻ�ѧ�������)__________________________________________________________��
�۵��������趨�ڸ����������ϵ�ԭ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ����ͼ��ʾ��
��1����ҺA�������� ��
��2����ⱥ��ʳ��ˮ�����ӷ���ʽ�� ��
��3�����ʱ�����������������Һ��pH��2��3���û�ѧƽ���ƶ�ԭ��������������� ��
��4��������õ���ˮ�辫�ơ�ȥ����Ӱ���Ca2����Mg2����NH4����SO42��[c(SO42����c(Ca2��)]��������������(����ˮ����ҺA���Ե���)�� 
������a����ɳ�⣬�����е������� ��
�ڹ��̢��н�NH4��ת��ΪN2�����ӷ���ʽ��
��BaSO4���ܽ�ȱ�BaCO3��С�����̢��г�ȥ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com