
·ÖĪö ÓÉ¢ŁŹµŃéæÉÖŖ£¬ĻņµŚŅ»·ŻČÜŅŗÖŠ¼ÓČėAgNO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś£¬°×É«³ĮµķĪŖAgCl»ņĢ¼ĖįŅų”¢ĮņĖįŅų£»
ÓÉ¢ŚæÉÖŖ£¬ĻņµŚ¶ž·ŻČÜŅŗÖŠ¼ÓČė×ćĮæNaOH ČÜŅŗŗó¼ÓČČ£¬ŹÕ¼Æµ½0.896L£Ø±ź×¼×“æö£©ĘųĢ壬ĘųĢåĪŖ°±Ęų£¬ĘäĪļÖŹµÄĮæĪŖ$\frac{0.896L}{22.4L/mol}$=0.04mol£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬NH4+£»
ÓÉ¢ŪæÉÖŖ£¬ĻņµŚČż·ŻČÜŅŗÖŠ¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬µĆµ½³Įµķ6.27g£¬³Įµķ¾×ćĮæŃĪĖįĻ“µÓŗóŹ£Óą2.33g£¬Ōņŗ¬SO42-µÄĪļÖŹµÄĮæĪŖ$\frac{2.33g}{233g/mol}$=0.01mol£¬ŗ¬CO32-µÄĪļÖŹµÄĮæĪŖ$\frac{6.27g-2.33g}{197g/mol}$=0.02mol£¬½įŗĻĄė×Ó¹²“ę”¢µēŗÉŹŲŗć½ā“š£®
½ā“š ½ā£ŗÓÉ¢ŁŹµŃéæÉÖŖ£¬ĻņµŚŅ»·ŻČÜŅŗÖŠ¼ÓČėAgNO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś£¬°×É«³ĮµķĪŖAgCl»ņĢ¼ĖįŅų”¢ĮņĖįŅų£»
ÓÉ¢ŚæÉÖŖ£¬ĻņµŚ¶ž·ŻČÜŅŗÖŠ¼ÓČė×ćĮæNaOH ČÜŅŗŗó¼ÓČČ£¬ŹÕ¼Æµ½0.896L£Ø±ź×¼×“æö£©ĘųĢ壬ĘųĢåĪŖ°±Ęų£¬ĘäĪļÖŹµÄĮæĪŖ$\frac{0.896L}{22.4L/mol}$=0.04mol£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬NH4+£»
ÓÉ¢ŪæÉÖŖ£¬ĻņµŚČż·ŻČÜŅŗÖŠ¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬µĆµ½³Įµķ6.27g£¬³Įµķ¾×ćĮæŃĪĖįĻ“µÓŗóŹ£Óą2.33g£¬Ōņŗ¬SO42-µÄĪļÖŹµÄĮæĪŖ$\frac{2.33g}{233g/mol}$=0.01mol£¬ŗ¬CO32-µÄĪļÖŹµÄĮæĪŖ$\frac{6.27g-2.33g}{197g/mol}$=0.02mol£¬ÓÉĄė×Ó¹²“ęæÉÖŖ£¬Ņ»¶Ø²»“ęŌŚFe3+”¢Mg2+£¬ÓɵēŗÉŹŲŗćæÉÖŖ£¬0.01mol”Į2+0.02mol”Į2£¾0.04mol£¬æÉÖŖŅ»¶Øŗ¬ŃōĄė×ÓK+£¬²»ÄÜČ·¶ØŹĒ·ńŗ¬Cl-£¬
£Ø1£©ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬Ņ»¶Ø“ęŌŚK+”¢NH4+”¢CO32-”¢SO42-£¬Ņ»¶Ø²»“ęŌŚFe3+”¢Mg2+£¬¹Ź“š°øĪŖ£ŗK+”¢NH4+”¢CO32-”¢SO42-£»Fe3+”¢Mg2+£»
£Ø2£©ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬æÉÄÜ“ęŌŚCl-£»ĪŖČ·¶ØøĆĄė×ÓŹĒ·ń“ęŌŚ£¬æÉȔɣĮæŌŹŌŅŗ£¬Č»ŗó¼ÓČė×ćĮæµÄĻ”ĻõĖįŗĶĻõĖį±µČÜŅŗ£¬¹żĀĖ£¬ĻņĀĖŅŗÖŠµĪČėAgNO3ČÜŅŗ£¬ČōÓŠ°×É«³ĮµķÉś³É£¬ŌņÖ¤Ć÷ÓŠCl-“ęŌŚ£¬¹Ź“š°øĪŖ£ŗCl-£»¼ÓČė×ćĮæµÄĻ”ĻõĖįŗĶĻõĖį±µČÜŅŗ£¬¹żĀĖ£¬ĻņĀĖŅŗÖŠµĪČėAgNO3ČÜŅŗ£¬ČōÓŠ°×É«³ĮµķÉś³É£¬ŌņÖ¤Ć÷ÓŠCl-“ęŌŚ£»
£Ø3£©ŌČÜŅŗÖŠ£¬æĻ¶Ø“ęŌŚµÄø÷Ąė×ÓµÄĪļÖŹµÄĮæÅØ¶Č·Ö±šĪŖ£ŗC£ØCO32-£©=$\frac{0.02mol}{0.1L}$=0.2mol/L£»C£ØSO42-£©=$\frac{0.01mol}{0.1L}$=0.1mol/L£»C£ØNH4+£©=$\frac{0.04mol}{0.1L}$=0.4mol/L£»ŅĄ¾ŻČÜŅŗÖŠµēŗÉŹŲŗćµĆµ½£ŗC£ØK+£©+C£ØNH4+£©=2C£ØSO42-£©+2C£ØCO32-£©£»C£ØK+£©=0.2mol/L£¬ČōČÜŅŗÖŠŗ¬ÓŠĀČĄė×Ó£¬ŌņC£ØK+£©£¾0.2mol/L£»
¹Ź“š°øĪŖ£ŗC£ØCO32-£©=0.2mol/L”¢C£ØSO42-£©=0.1mol/L£»C£ØNH4+£©=0.4mol/L”¢C£ØK+£©”Ż0.2mol/L£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄ¼ģŃé¼°ĶʶĻ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĻÖĻóÓė·“Ó¦”¢Ąė×ÓĶʶĻµÄ¹ŲĻµĪŖ½ā“šµÄ¹Ų¼ü£¬ŹµŃé¢óÖŠ°×É«³ĮµķĪŖ½ā“šµÄĶ»ĘĘæŚ£¬²ąÖŲ·ÖĪöÓė¼ĘĖć”¢ĶʶĻÄÜĮ¦µÄ漲飬עŅāµēŗÉŹŲŗćµÄÓ¦ÓĆ£¬ĢāÄæÄѶČÖŠµČ£®


 ŹżŃ§°ĀČüŹī¼ŁĢģĢģĮ·ÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŹżŃ§°ĀČüŹī¼ŁĢģĢģĮ·ÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø ÄĻ“ó½ĢøØĒĄĻČĘšÅÜŹī¼ŁĻĪ½Ó½Ģ³ĢÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ÄĻ“ó½ĢøØĒĄĻČĘšÅÜŹī¼ŁĻĪ½Ó½Ģ³ĢÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
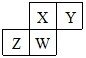 ¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢WŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠWŌ×ÓµÄÖŹ×ÓŹżŹĒĘä×īĶā²ćµē×ÓŹżµÄČż±¶£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢WŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠWŌ×ÓµÄÖŹ×ÓŹżŹĒĘä×īĶā²ćµē×ÓŹżµÄČż±¶£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | Ō×Ó°ė¾¶£ŗZ£¾W£¾X£¾Y | |
| B£® | ŌŖĖŲX”¢Y”¢Z”¢WµÄ×īøß»ÆŗĻ¼Ū·Ö±šÓėĘäÖ÷×åŠņŹżĻąµČ | |
| C£® | ×ī¼ņµ„ĘųĢ¬Ēā»ÆĪļµÄČČĪČ¶ØŠŌ£ŗY£¾X£¾W£¾Z | |
| D£® | ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ£ŗX£¾W£¾Z |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
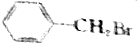 ¢ŚCH3CH2CH3Br ¢ŪCH3Br ¢ÜCH3CHBrCH2CH3
¢ŚCH3CH2CH3Br ¢ŪCH3Br ¢ÜCH3CHBrCH2CH3²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹čĖįÄĘČÜŅŗÖŠĶØČė¹żĮæµÄCO2£ŗSiO32-+CO2+H2OØTH2SiO3”ż+CO32- | |
| B£® | ½«FeCl3±„ŗĶČÜŅŗµĪČė·ŠĖ®ÖŠÖĘČ”Fe£ØOH£©3½ŗĢå£ŗFe3++3H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$Fe£ØOH£©3£Ø½ŗĢ壩+3H+ | |
| C£® | ĻņNaHSO4ČÜŅŗÖŠ¼ÓČė¹żĮæµÄBa£ØOH£©2ČÜŅŗ£ŗBa2++2OH-+2H++SO42-ØTBaSO4”ż+2H2O | |
| D£® | Ļņ0.1mol/L”¢pH=1µÄNaHAČÜŅŗÖŠ¼ÓČėNaOHČÜŅŗ£ŗHA-+OH-ØTA2-+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
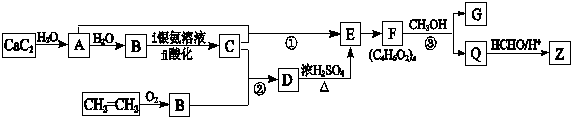
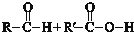 $\stackrel{Ņ»¶ØĢõ¼ž}{”ś}$
$\stackrel{Ņ»¶ØĢõ¼ž}{”ś}$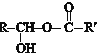
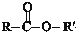 $?_{”÷}^{“߻ƼĮ}$
$?_{”÷}^{“߻ƼĮ}$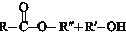
 £®
£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
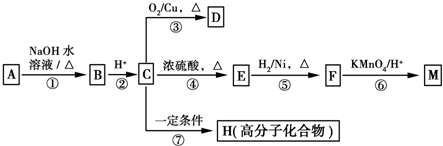
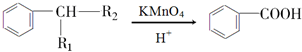 £ØR1”¢R2±ķŹ¾Ģž»ł»ņĒāŌ×Ó£©
£ØR1”¢R2±ķŹ¾Ģž»ł»ņĒāŌ×Ó£©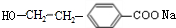 £®
£®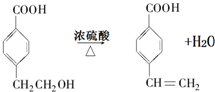 £®
£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ü | B£® | ¢Ś¢Ü | C£® | ¢Ū¢Ż | D£® | ¢Ü¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĻ©µÄ½į¹¹Ź½£ŗC2H4 | B£® | ¼×Ķé·Ö×ӵıȥżÄ£ŠĶ£ŗ | ||
| C£® | ĖÄĀČ»ÆĢ¼µÄµē×ÓŹ½£ŗ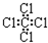 | D£® | ±½µÄ·Ö×ÓŹ½£ŗ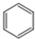 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com