
”¾ĢāÄæ”æijŠ©·Ļ¾ÉĖÜĮĻæɲÉÓĆĻĀĮŠ·½·Ø“¦Ąķ£ŗ½«·ĻĖÜĮĻøō¾ųæÕĘų¼ÓĒæČČ£¬Ź¹Ęä±ä³ÉÓŠÓƵÄĪļÖŹ£¬ŹµŃé×°ÖĆČēĻĀĶ¼”£¼ÓČČ¾Ū±ūĻ©·ĻĖÜĮĻµĆµ½µÄ²śĪļČēĻĀ±ķ£ŗ
²śĪļ | ĒāĘų | ¼×Ķé | ŅŅĻ© | ±ūĻ© | ±½ | ¼×±½ | Ģ¼ |
·Šµć£Ø”ę£© | -252£®8 | -146 | -103£®7 | -47£®4 | 80£®10 | 110£®63 | 4827 |

£Ø1£©¼×ŹŌ¹ÜÖŠ×īÖÕ²ŠĮōĪļŹĒ_____________”£ĖüÓŠ¶ąÖÖÓĆĶ¾£¬ČēĻĀĮŠ×Ŗ»Æ¾ĶæÉÖĘČ”¾ŪŅŅČ²”£Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½__________________________ ”£
![]()
£Ø2£©ŅŅÖŠŹŌ¹ÜŹÕ¼Æµ½µÄĮ½ÖÖ²śĘ·ÖŠ£¬ÓŠŅ»ÖÖÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«µÄĪļÖŹ£¬øĆĪļÖŹĪŖ__________”£
£Ø3£©±ū֊׶ŠĪĘæ¹Ū²ģµ½µÄĻÖĻó_____________________________________”£·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ__________________________”¢_________________________”£
£Ø4£©¾äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ³ä·ÖĪüŹÕ£¬×īŗóŹÕ¼Æµ½µÄĘųĢåŹĒ______________”£
”¾“š°ø”æC»ņĢ¼ CaC2 +2H2O ”ś Ca (OH)2 + C2H2”ü ¼×±½ ČÜŅŗĶŹÉ« CH2=CH2+Br2”śCH2BrCH2Br CH3CH=CH2£«Br2”śCH3CHBrCH2Br ¼×Ķ锢ĒāĘų
”¾½āĪö”æ
(1). ÓɱķÖŠø÷²śĪļµÄ·ŠµćŹż¾ŻæÉÖŖ£¬¾Ū±ūĻ©·ĻĖÜĮĻ¼ÓĒæČČŹ±¼×ŹŌ¹ÜÖŠµÄ²ŠĮōĪļĪŖC£¬µēŹÆµÄÖ÷ŅŖ³É·ÖŹĒĢ¼»ÆøĘ£¬Ģ¼»ÆøĘÓėĖ®·“Ӧɜ³ÉĒāŃõ»ÆøĘŗĶŅŅČ²£»
(2). ŅŅÖŠŹŌ¹ÜÓĆĄäĖ®µĆµ½µÄĮ½ÖÖ²śĘ·ĪŖ±½ŗĶ¼×±½£¬¼×±½æÉŅŌŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£»
(3). ŅŅĻ©”¢±ūĻ©æÉŅŌŗĶäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·¢Éś¼Ó³É·“Ó¦£»
(4). ¾¹żÉĻŹö¹ż³Ģ“¦Ąķŗó£¬×īŗóŹÕ¼Æµ½µÄĘųĢåŹĒ¼×ĶéŗĶĒāĘų”£
(1). ¾Ū±ūĻ©·ĻĖÜĮĻ¼ÓĒæČČŹ±µĆµ½µÄ²śĪļÓŠ£ŗĒāĘų”¢¼×Ķ锢ŅŅĻ©”¢±ūĻ©”¢±½”¢¼×±½ŗĶĢ¼£¬ÓɱķÖŠ·ŠµćŹż¾ŻæÉÖŖ£¬¼×ŹŌ¹ÜÖŠ×īÖÕ²ŠĮōĪļŹĒC£¬µēŹÆµÄÖ÷ŅŖ³É·ÖŹĒĢ¼»ÆøĘ£¬Ģ¼»ÆøĘÓėĖ®·“Ӧɜ³ÉĒāŃõ»ÆøĘŗĶŅŅČ²£¬»Æѧ·½³ĢŹ½ĪŖ£ŗCaC2 +2H2O ”ś Ca (OH)2 + C2H2”ü£¬¹Ź“š°øĪŖ£ŗC»ņĢ¼£»CaC2 +2H2O ”ś Ca (OH)2 + C2H2”ü£»
(2). øł¾Ż²śĪļµÄ·ŠµćæÉÖŖ£¬ŅŅÖŠÓĆĄäĖ®ĄäČ“ŗóµĆµ½µÄ²śĘ·ŹĒ±½ŗĶ¼×±½£¬±½²»ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬¼×±½æÉŅŌŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬¹Ź“š°øĪŖ£ŗ¼×±½£»
(3). “ÓŅŅÖŠ³öĄ“µÄ²śĪļÖŠŗ¬ÓŠŅŅĻ©ŗĶ±ūĻ©£¬¶žÕ߶¼æÉŅŌŗĶäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·¢Éś¼Ó³É·“Ó¦Ź¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ«£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCH2=CH2+Br2”śCH2BrCH2Br£¬CH3CH=CH2£«Br2”śCH3CHBrCH2Br£¬¹Ź“š°øĪŖ£ŗČÜŅŗĶŹÉ«£»CH2=CH2+Br2”śCH2BrCH2Br£»CH3CH=CH2£«Br2”śCH3CHBrCH2Br£»
(4). ¾¹żÉĻŹö¹ż³Ģ“¦Ąķŗó£¬×īŗóŹÕ¼Æµ½µÄĘųĢåŹĒ¼×ĶéŗĶĒāĘų£¬¹Ź“š°øĪŖ£ŗ¼×Ķ锢ĒāĘų”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČżĮņ“śĢ¼ĖįÄĘ(Na2CS3)³£ÓĆ×÷ɱ¾ś¼Į”¢³Įµķ¼Į”£Ä³Š”×éÉč¼ĘŹµŃéĢ½¾æČżĮņ“śĢ¼ĖįÄʵĊŌÖŹ²¢²ā¶ØĘäČÜŅŗµÄÅØ¶Č”£
ŹµŃé1£ŗĢ½¾æNa2CS3µÄŠŌÖŹ
²½Öč | ²Ł×÷¼°ĻÖĻó |
¢Ł | ȔɣĮæNa2CS3¹ĢĢåČÜÓŚÕōĮóĖ®ÅäÖĘ³ÉČÜŅŗ²¢·Ö³ÉĮ½µČ·Ż |
¢Ś | ĻņĘäÖŠŅ»·ŻČÜŅŗÖŠµĪ¼Ó¼øµĪ·ÓĢŖŹŌŅŗ£¬ČÜŅŗ±äŗģÉ« |
¢Ū | ĻņĮķŅ»·ŻČÜŅŗÖŠµĪ¼ÓĖįŠŌKMnO4ČÜŅŗ£¬×ĻÉ«ĶŹČ„ |
£Ø1£©H2CS3ŹĒ________Ėį£ØĢī”°Ēæ”±»ņ”°Čõ”±£©”£
£Ø2£©ŅŃÖŖ²½Öč¢ŪµÄŃõ»Æ²śĪļŹĒSO42£,Š“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½______
£Ø3£©Ä³Ķ¬Ń§Č”²½Öč¢ŪĖłµĆČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼Ó×ćĮæŃĪĖį”¢BaCl2ČÜŅŗ²śÉś°×É«³Įµķ£¬ĖūČĻĪŖĶعż²ā¶Ø²śÉśµÄ°×É«³ĮµķµÄÖŹĮ漓æÉĒó³öŹµŃéĖłÓĆNa2CS3µÄĮ棬ÄćŹĒ·ńĶ¬ŅāĖūµÄ¹Ūµć²¢ĖµĆ÷ĄķÓÉ______”£
ŹµŃé2£ŗ²ā¶ØNa2CS3ČÜŅŗµÄÅضČ

°“ČēĶ¼ĖłŹ¾Į¬½ÓŗĆ×°ÖĆ£¬Č”100mLNa2CS3ČÜŅŗÖĆÓŚČż¾±ÉÕĘæÖŠ£¬“ņæŖŅĒĘ÷dµÄ»īČū£¬µĪČė×ćĮæ2.0mol/LĻ”H2SO4£¬¹Ų±Õ»īČū”£
ŅŃÖŖ£ŗNa2CS3 + H2SO4=Na2SO4 + CS2 + H2S”ü”£CS2ŗĶH2S¾łÓŠ¶¾”£CS2²»ČÜÓŚĖ®£¬·Šµć46”ę£¬ÓėCO2ijŠ©ŠŌÖŹĻąĖĘ£¬ÓėNaOH×÷ÓĆÉś³ÉNa2COS2ŗĶH2O”£
£Ø4£©Ź¢·ÅĪŽĖ®CaCl2µÄŅĒĘ÷µÄĆū³ĘŹĒ______£¬BÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ______”£
£Ø5£©·“Ó¦½įŹųŗó“ņæŖ»īČūk£¬ŌŁ»ŗĀżĶØČėČČN2Ņ»¶ĪŹ±¼ä£¬ĘäÄæµÄŹĒ______”£
£Ø6£©ĪŖĮĖ¼ĘĖćNa2CS3ČÜŅŗµÄÅØ¶Č£¬¶ŌBÖŠ»ģŗĻĪļ½ųŠŠ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘÖŲ£¬µĆ19.2g¹ĢĢ壬ŌņAÖŠNa2CS3µÄĪļÖŹµÄĮæÅضČĪŖ______”£
£Ø7£©·ÖĪöÉĻŹöŹµŃé·½°ø£¬»¹æÉŅŌĶعż²ā¶ØCÖŠČÜŅŗÖŹĮæµÄŌö¼ÓÖµĄ“¼ĘĖćNa2CS3ČÜŅŗµÄÅØ¶Č£¬Čō·“Ó¦½įŹųŗó½«ĶØČČN2øÄĪŖĶØČČæÕĘų£¬¼ĘĖćÖµ______£ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÖŠŅ½µä¼®”¶Öāŗó±ø¼±·½”·ĖłŌŲÖĪĮĘű¼²µÄ·½·Ø”°ĒąŻļŅ»ĪÕ£¬ŅŌĖ®¶žÉż×Õ£¬½ŹČ”Ö£¬¾”·žÖ®”±£¬ ¶ŌĶĄßĻßĻĶŶÓŃŠ·¢ÖĪĮĘű¼²µÄĢŲŠ§Ņ©”Ŗ”ŖĒąŻļĖŲÓŠ¾Ž“óµÄĘōŹ¾×÷ÓĆ”£ĶĄßĻßĻŌŚ¶Ō±ČŹµŃéÖŠ·¢ĻֹŷØĮĘŠ§µĶĻĀ£¬ĖżÉč¼ĘµÄĢįČ”ĒąŻļĖŲµÄ¹¤ŅÕæÉŅŌ¼ņ»Æ³ÉČēĻĀĮ÷³Ģ£ŗ
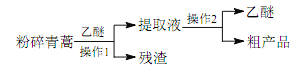
øł¾ŻÉĻŹöŠÅĻ¢·ÖĪö£¬ĻĀĮŠÅŠ¶Ļ»ņŠšŹöÖŠæĻ¶Ø“ķĪóµÄŹĒ
A. “ÓĢģČ»Ö²ĪļÖŠĢįȔӊŠ§³É·ÖÖĪĮĘ¼²²”ŹĒŅ©ĪļŃŠ·¢µÄÖŲŅŖĶ¾¾¶
B. ½«ĒąŻļ·ŪĖéæÉŅŌĢįøßÓŠŠ§³É·ÖµÄĢįČ”ĀŹ
C. ĒąŻļĖŲŅ×ČÜÓŚĖ®ŗĶŅŅĆŃ
D. ²Ł×÷ 1 ŹĒ¹żĀĖ£¬²Ł×÷ 2 ŹĒÕōĮó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖ£ŗA”¢B”¢C”¢D”¢EĪåÖÖŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪŌö“ó”£AŹĒ¶ĢÖÜĘŚÖŠŌ×Ó°ė¾¶×ī“óµÄŌŖĖŲ£¬BŌŖĖŲ3pÄܼ¶°ė³äĀś£»CŹĒĖłŌŚÖÜĘŚµēøŗŠŌ×ī“óµÄŌŖĖŲ£»DŹĒµŚĖÄÖÜĘŚĪ“³É¶Ōµē×Ó×ī¶ąµÄŌŖĖŲ£»EµÄŌ×ÓŠņŹż±ČD“ó3”£ŹŌ»Ų“šĻĀĮŠÓŠ¹ŲµÄĪŹĢā£ŗ
£Ø1£©Š“³öDŌŖĖŲ¼Ūµē×ӵĵē×ÓÅŲ¼Ķ¼£ŗ______________”£
£Ø2£©DæÉŠĪ³É»ÆŗĻĪļ[D(H2O)6](NO3)3 ,[D(H2O)6](NO3)3ÖŠŅõĄė×ÓµÄĮ¢Ģå¹¹ŠĶŹĒ____________”£NO2£ÖŠŠÄŌ×ӵĹģµĄŌÓ»ÆĄąŠĶĪŖ______________£¬1 mol [D(H2O)6] 3+ ÖŠŗ¬ÓŠµÄ¦Ņ¼üŹżĪŖ ______________”£
£Ø3£©ŅŃÖŖB”¢CĮ½ÖÖŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļĶس£ÓŠĮ½ÖÖ”£ÕāĮ½ÖÖ»ÆŗĻĪļÖŠ___________ £ØĢī»ÆѧŹ½£©ĪŖ·Ē¼«ŠŌ·Ö×Ó”£ĮķŅ»ÖÖĪļÖŹµÄµē×ÓŹ½ĪŖ ______________”£
£Ø4£©DŹĒŅ»ÖÖÓ²¶ų“ąæ¹øÆŹ“ŠŌĒæµÄ½šŹō£¬³£ÓĆÓŚµē¶ĘŗĶÖĘŌģĢŲÖÖøÖ”£ĻĀĶ¼ĪŖDµÄ¾§°ū½į¹¹Ķ¼£¬ŌņD¾§°ūŹōÓŚ___________¶Ń»ż£»øĆ¾§°ūÖŠŌ×ÓµÄĢå»żÕ¼¾§°ūĢå»żµÄ°Ł·ÖĀŹĪŖ___________”££ØŅŃÖŖ£ŗ¦Š=3.14£¬![]() =1.732£©
=1.732£©

£Ø5£©E¾§ĢåµÄŅ»ÖÖ¾§°ū(ČēĶ¼ĖłŹ¾)µÄ±ß³¤ĪŖanm£¬ĆܶČĪŖ¦Ńg”¤cm£3£¬NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ŌņEµÄŌ×Ó°ė¾¶ĪŖ___________nm£¬EµÄĻą¶ŌŌ×ÓÖŹĮææɱķŹ¾ĪŖ___________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠ»śĪļWÓĆ×÷µ÷Ļć¼Į”¢øß·Ö×Ó²ÄĮĻŗĻ³ÉµÄÖŠ¼äĢåµČ£¬ÖʱøWµÄŅ»ÖÖŗĻ³ÉĀ·ĻßČēĻĀ”£
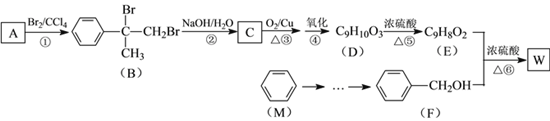
ŅŃÖŖ£ŗ![]()
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©FµÄ»ÆѧĆū³ĘŹĒ_________£¬¢ŚµÄ·“Ó¦ĄąŠĶŹĒ_________”£
£Ø2£©DÖŠŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ________________£ØŠ“Ćū³Ę£©£¬D¾ŪŗĻÉś³Éøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ĪŖ_____________”£
£Ø3£©·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ŹĒ______________________”£
£Ø4£©·“Ó¦¢ŽµÄ»Æѧ·½³ĢŹ½ŹĒ______________________”£
£Ø5£©·¼Ļć»ÆŗĻĪļNŹĒAµÄĶ¬·ÖŅģ¹¹Ģ壬ĘäÖŠŗĖ“Ź²ÕńĒāĘ×ĪŖČż×é·åµÄ½į¹¹¼ņŹ½ĪŖ
_______________ӣ
£Ø6£©²ĪÕÕÓŠ»śĪļWµÄÉĻŹöŗĻ³ÉĀ·Ļߣ¬Éč¼ĘŅŌMĪŖĘšŹ¼ŌĮĻÖʱøFµÄŗĻ³ÉĀ·Ļß(ĪŽ»śŹŌ¼ĮČĪŃ”)”£[Ź¾Ąż£ŗ![]() ]
]
____________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĢĒĄąŹĒÓÉ __”¢____”¢ __ ČżÖÖŌŖĖŲ×é³ÉµÄŅ»ĄąÓŠ»ś»ÆŗĻĪļ£¬ĢĒĄąÓÖ½Š×ö__£¬ŌŅņŹĒĖüĆĒµÄ»Æѧ×é³É“󶹏ż·ūŗĻĶØŹ½____”£µ«²»ŹĒĖłÓŠµÄĢĒ·ūŗĻÕāøöĶØŹ½ĒŅ·ūŗĻÕāøöĶØŹ½µÄŅ²²»Ņ»¶ØŹĒĢĒ”£ÓĶÖ¬µÄ³É·ÖŹĒ________£¬ÓÉ__×é³É£¬½į¹¹¼ņŹ½ĪŖ________”£Ī¬ÉśĖŲŹĒ²ĪÓėÉśĪļ ___ŗĶ ______Ėł±ŲŠčµÄŅ»ĄąŠ”·Ö×ÓÓŠ»ś»ÆŗĻĪļ£¬Ėü·ÖĪŖ____Ī¬ÉśĖŲŗĶ____Ī¬ÉśĖŲ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ĪŖŹµŃéŹŅijÅØŃĪĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©µÄÓŠ¹ŲÄŚČŻ£¬ŹŌøł¾Ż±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©½«±ź×¼×“æöĻĀ___LĀČ»ÆĒāĘųĢåĶØČė1.00LĖ®ÖŠæɵĆ36.5%µÄÅØŃĪĖį£¬øĆÅØŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČĪŖ___mol”¤L£1”£
£Ø2£©Č”ÓĆČĪŅāĢå»żµÄøĆŃĪĖįŹ±£¬ĻĀĮŠĪļĄķĮæÖŠ²»ĖęĖłČ”Ģå»żµÄ¶ąÉŁ¶ų±ä»ÆµÄŹĒ_____”£
A£®ČÜŅŗÖŠHClµÄĪļÖŹµÄĮæ B£®ČÜŅŗµÄÅØ¶Č C£®ČÜŅŗÖŠCl£µÄŹżÄæ D£®ČÜŅŗµÄĆܶČ
£Ø3£©Ä³Ń§ÉśÓūÓĆÉĻŹöÅØŃĪĖįŗĶÕōĮóĖ®ÅäÖĘ500mLĪļÖŹµÄĮæÅضČĪŖ0.400mol/LµÄĻ”ŃĪĖį”£
¢ŁøĆѧɜŠčŅŖĮæČ”___mLÉĻŹöÅØŃĪĖį½ųŠŠÅäÖĘ”£
¢ŚÅäÖĘŅĒĘ÷³żÉÕ±”¢ĮæĶ²ŗĶ²£Į§°ō£¬»¹ŠčŅŖµÄŅĒĘ÷ŹĒ___”¢____”£
¢ŪÅäÖĘŹ±£¬ĻĀĮŠ²Ł×÷ÕżČ·µÄĖ³ŠņŹĒ£ØÓĆ×ÖÄø±ķŹ¾£©____”£
A.Ļ“µÓ B.¶ØČŻ C.Čܽā D.Ņ”ŌČ E.ĄäČ“ F.³ĘĮæ G.×ŖŅĘ
¢ÜŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠŹµŃé²Ł×÷¶ŌĖłÅäÖʵÄĻ”ŃĪĖįµÄĪļÖŹµÄĮæÅضČÓŠŗĪÓ°Ļģ£æ(ŌŚĄØŗÅÄŚĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)”£
¢ń.Ī“µČĻ”ŹĶŗóµÄČÜŅŗĄäČ“µ½ŹŅĪĀ¾Ķ×ŖŅʵ½ČŻĮæĘæ£Ø______£©
¢ņ.¶ØČŻŗó¾Õńµ“”¢Ņ”ŌČ”¢¾²ÖĆ£¬·¢ĻÖŅŗĆęĻĀ½µ£¬ŌŁ¼ÓŹŹĮæµÄÕōĮóĖ®”££Ø______£©
¢ó.ÅäÖĘŹ±£¬ČŻĮæĘæÓŠÉŁĮæÕōĮóĖ®£Ø______£©
¢ō.¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß”££Ø_______£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĀČ»ÆŃĒĶ(CuCl)ŹĒÓŠ»śŗĻ³É¹¤ŅµÖŠÓ¦ÓĆ½Ļ¹ć·ŗµÄ“߻ƼĮ£¬¼ū¹āŅ×·Ö½ā£¬ŌŚ³±ŹŖæÕĘųÖŠŅ×±»Ńõ»Æ£¬ÄŃČÜÓŚĖ®”£
(1)ŹµŃéŹŅ±£“ęŠĀÖĘCuCl¾§ĢåµÄ·½·ØŹĒ_________________”£
(2)ŅŃÖŖ£ŗCu(s)+Cl2(g)=CuCl2(s) ”÷H1=-218.8kJ/mol
2Cu(s)+O2(g)=2CuO(s) ”÷H2=-310.6kJ/mol
2CuCl2(s)=2CuCl(s)+Cl2(g) ”÷H3=+152.3kJ/mol
Ōņ·“Ó¦4CuCl(s)+O2(g)=2CuCl2(s)+2CuO(s)µÄ”÷H=____kJ/mol”£
(3)ĄūÓĆCuClÄŃČÜÓŚĖ®µÄŠŌÖŹ£¬æÉŅŌ³żČ„·ĻĖ®ÖŠµÄCl-”£Ļņŗ¬Cl-µÄ·ĻĖ®ÖŠĶ¬Ź±¼ÓČėCuŗĶCuSO4£¬ČÜŅŗÖŠĘ½ŗāŹ±Ļą¹ŲĄė×ÓÅØ¶Č¹ŲĻµČēĶ¼ĖłŹ¾”£[ŅŃÖŖ£ŗpc(Ąė×Ó)=£lgc(Ąė×Ó)]

¢Ł³żČ„·ĻĖ®ÖŠCl-µÄĄė×Ó·½³ĢŹ½ĪŖ_________________”£
¢ŚKsp(CuCl)=___________”£
¢ŪŅŃÖŖ£ŗCu+Cu2+![]() 2Cu+ K=7.6”Į10-7”£Ķعż¼ĘĖćĖµĆ÷ÉĻŹö³żCl-µÄ·“Ó¦ÄÜĶźČ«½ųŠŠµÄŌŅņ______________________________”£
2Cu+ K=7.6”Į10-7”£Ķعż¼ĘĖćĖµĆ÷ÉĻŹö³żCl-µÄ·“Ó¦ÄÜĶźČ«½ųŠŠµÄŌŅņ______________________________”£
(4)T”ꏱ£¬ÓĆH2»¹ŌCuClÖʱø»īŠŌĶ£ŗH2(g)+2CuCl(s)![]() 2Cu(s)+2HCl(g)£¬“ļµ½Ę½ŗāŹ±£¬H2µÄ×Ŗ»ÆĀŹ(
2Cu(s)+2HCl(g)£¬“ļµ½Ę½ŗāŹ±£¬H2µÄ×Ŗ»ÆĀŹ(![]() )ĪŖ80%”£·“Ó¦ĖŁĀŹv=vÕż£vÄę=
)ĪŖ80%”£·“Ó¦ĖŁĀŹv=vÕż£vÄę=![]() £¬kÕż”¢kÄę·Ö±šĪŖÕż”¢Äę·“Ó¦ĖŁĀŹ³£Źż£¬xĪŖĘųĢåµÄĪļÖŹµÄĮæ·ÖŹż”£µ±
£¬kÕż”¢kÄę·Ö±šĪŖÕż”¢Äę·“Ó¦ĖŁĀŹ³£Źż£¬xĪŖĘųĢåµÄĪļÖŹµÄĮæ·ÖŹż”£µ±![]() =60£„Ź±£¬
=60£„Ź±£¬![]() =________(±£Įō1Ī»Š”Źż)”£
=________(±£Įō1Ī»Š”Źż)”£
(5)CuClČÜÓŚÅØ°±Ė®µÄ·“Ó¦ŌĄķĪŖCuCl+2NH3”¤H2O![]() [Cu(NH3)2]-+2H2O+Cl-£¬øĆ·“Ó¦æŲÖĘĪĀ¶ČĪŖ70”«80”ę£¬ĘäŌŅņŹĒ_______________________”£
[Cu(NH3)2]-+2H2O+Cl-£¬øĆ·“Ó¦æŲÖĘĪĀ¶ČĪŖ70”«80”ę£¬ĘäŌŅņŹĒ_______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃé²āµĆ25”ę”¢101 kPaŹ±£¬1 mol¼×“¼ĶźČ«Č¼ÉÕŹĶ·Å726.51kJµÄČČĮ棬ĻĀĮŠČČ»Æѧ·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ( )
A. 2CH3OH£«3O2£½2CO2£«4H2O””¦¤H£½£1453.02 kJ”¤mol£1
B. 2CH3OH(l)£«3O2(g)£½2CO2(g)£«4H2O(g)””¦¤H£½£«1453.02 kJ”¤mol£1
C. CH3OH(l)£«3/2O2(g)£½CO2(g)£«2H2O(l)””¦¤H£½£726.51 kJ”¤mol£1
D. CH3OH(l)£«3/2O2(g)£½CO2(g)£«2H2O(g)””¦¤H£½£726.51 kJ”¤mol£1
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com