
���������μ����ֽ��Ҳ���ϸ��ӡ�ijѧϰС����Mg(NO3)2Ϊ�о�������ͨ��ʵ��̽�����ȷֽ�IJ���������4�ֲ��룺
�ף�Mg(NO3)2��NO2��O2 �ң�MgO��NO2��O2
����Mg3N2��O2 ����MgO��NO2��N2
��1��ʵ��ǰ��С���Ա�������϶����붡�������������� ��
�������ϵ�֪��2NO2+2NaOH=NaNO3+NaNO2+H2O,��Լס��ҡ������룬�������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���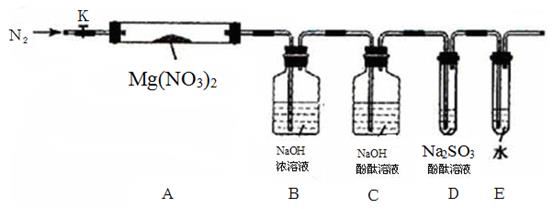
��2��ʵ�����
��ȡ�����Ӻ��˹����Լ�֮ǰ���ر�K����Ӳ�ʲ����ܣ�A�����۲쵽E �������������ų������� ��
�� ��ȡMg(NO3)2����3. 79 g����A�У�����ǰͨ��N2������װ���ڵĿ�������Ŀ����________���ر�K���þƾ��Ƽ���ʱ����ȷ��������______Ȼ��̶��ڹ��й��岿λ�¼��ȡ�
�� �۲쵽A ���к���ɫ������֣�C��D ��δ�����Ա仯��
�� ����Ʒ��ȫ�ֽ⣬A װ����ȴ�����¡����������ʣ����������Ϊ1.0g
�� ȡ����ʣ��������Թ��У���������ˮ��δ����������
��3��ʵ������������
�� ����ʵ�������ʣ�����������������ɳ���ȷ�ϲ���_______����ȷ�ġ�
�� ����D ������������һλͬѧ��Ϊ����ȷ�Ϸֽ��������O2����Ϊ����O2��D�н�����������ԭ��Ӧ ����д��ѧ����ʽ������Һ��ɫ����ȥ��С�������϶��ֽ��������O2���ڣ�δ��ൽ��ԭ���� ��
�� С�����ۺ��ɵĹ�ʶ������ʵ������Բ����ƣ���Ľ�װ���һ���о���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУѧ����ѧʵ��С�飬Ϊ��֤�ǽ���Ԫ�ط���������ǿ����͵��������һ��ʵ��װ�ã������ּӳ�װ������ȥ��
��1��д��A�з�Ӧ�����ӷ���ʽ ��
��2��B�г��ֻ�ɫ��������������������ӷ���ʽ ��
��3���Դ�ԭ�ӽṹ�ǶȽ����ȵ������Դ������ԭ�� ��
��4��D�и�����г��ֵ�����ѧ����ʽ ��
��5����ͬѧ��ΪD�е�������˵���ȵ������Դ��ڵ�����Ҫ��C֮ǰ��װϴ��װ�ã��뻭����װ��ͼ ����ע��ʢװ�Լ�����
��6������ʲô������֤��������Cl2��S����һ�������ʵ˵�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������Ũ�������ܷ����ۻ�����ij��ȤС���ͬѧ���ֽ�һ����������Ũ�������ʱ���۲쵽����ȫ�ܽ⣬�������������塣Ϊ�ˣ��������������װ����֤�����������塣
��1��֤����Ӧ����������������SO2���ɵ������� ��
��2��֤�������к���������ʵ�������� ��
��3��Ϊ�˽�һ��̽����Ӧ��A��Һ����Ԫ�صļ�̬�����ǽ��������µļ��裺
����1����Һ����Ԫ�ؼ���Fe3+Ҳ��Fe2+
����2����Һ����Ԫ��ֻ��Fe3+
����3����Һ����Ԫ��ֻ��________________
���ڼ���1�������Լ���0.01 mol/L����KMnO4��Һ��ϡ��ˮ��Һ��0.1 mal/L KI��Һ��
������Һ��KSCN��Һ������ˮ����̽����������Һ������ɱ������ݡ�
��ʵ��̽����
| ʵ����� | Ԥ������ | ���� |
| ȡ��Ӧ���A��Һ��װ��a��b���Թܣ�����٣���a�Թ��е��� �� | | |
| ����ڣ���b�Թ��е��� �� | | ��Һ����Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ̽����Ȳ����ļӳɷ�Ӧ����ͬѧ��Ʋ�����������ʵ�飺��ȡһ������ҵ�õ�ʯ��ˮ��Ӧ�������ɵ�����ͨ����ˮ�У�������Һ��ɫ����֤����Ȳ����ˮ�����˼ӳɷ�Ӧ��
��ͬѧ�����ڼ�ͬѧ��ʵ���У���ɫ�����Һ������������ɫ���ǣ��Ʋ����Ƶõ���Ȳ�л����ܺ���������ԭ�Ե��������壬�ɴ�����������ȳ�ȥ֮��������ˮ��Ӧ��
����ش��������⣺
��1��д����ͬѧʵ����������Ҫ�Ļ�ѧ����ʽ�� __________________________ ��
_________________________________ ��
��2�����ݼ�ͬѧ��Ƶ�ʵ�鼰������֤������Ȳ���巢���˼ӳɷ�Ӧ���������� _______
��a��ʹ��ˮ��ɫ�ķ�Ӧ��δ���Ǽӳɷ�Ӧ ��b��ʹ��ˮ��ɫ�ķ�Ӧ�����Ǽӳɷ�Ӧ
��c��ʹ��ˮ��ɫ�����ʣ�δ������Ȳ ��d��ʹ��ˮ��ɫ�����ʣ�������Ȳ
��3����ͬѧ�Ʋ����Ȳ�бض����е�һ������������ ��������ˮ��Ӧ�����ӷ���ʽ�� ������֤�����б���ȫ����ȥ��
��4��Ϊ��֤�˷�Ӧ�Ǽӳɶ�����ȡ������ͬѧ��pH��ֽ�����Է�Ӧ����Һ�����ԣ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��ҵ�ϳ�����������ʢװ��Ũ���ᣬΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧУѧϰС���������̽��ʵ�飺
[һ]��1�����ѳ�ȥ�����������������̼�ظ֣���������ȵ�Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2������ȡ����6.0g����15.0mLŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y���ټ�ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����е�Fe2+��Ӧѡ��
��ѡ����ţ�
a.KSCN��Һ����ˮ����.b��.�ۺ�KSCN��Һ������c.Ũ��ˮ����d.����KMnO4��Һ
����ͬѧȡ336mL����״��������Yͨ��������ˮ�У�������ӦSO2+Br2+2H2O�T2HBr+H2SO4��Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33g���ڴ���֪����Y��SO2���������Ϊ ��
[̽����]��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ�����H2��Q���壬Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�����ʡ�ԣ�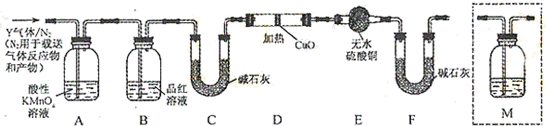
��3��װ��B���Լ��������� ��
��4����Ϊ����Y�л�����Q�������� �����û�ѧ����ʽ��ʾ��
��5��Ϊȷ��Q�Ĵ��ڣ�����װ��������M�� ��ѡ����ţ�
a.A֮ǰ��������b.A-B�䡡������c.B-C�䡡������d.C-D��
��6���������Y�к���H2��Ԥ��ʵ�������Ӧ�� ��
��7����Ҫ�ⶨ���������Y��H2��������״����Լ��28ml��H2�������ò���H2��������⣬��ѡ���������������������жϲ�˵������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣�
̼����ĺ���Ӱ��������ܣ�̼��������һ�ֲⶨ�����ǽ�������̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
��1������װ��A���ڸ�����x�˸�����̼����ת��ΪCO2��SO2��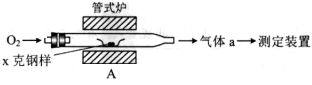
������a�ijɷ���____________________��
��������������FeS��ʽ���ڣ�A�з�Ӧ��3FeS+5 O2����1____+3_____��
��2��������aͨ�������װ���У�����ͼ�������õζ����ⶨ��ĺ�����
��H2O2����SO2�Ļ�ѧ����ʽ��_________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mLNaOH��Һ��������1mLNaOH��Һ�൱���������Ϊy�ˣ���ø������������������_________________��
��3��������aͨ���̼װ���У�����ͼ���������������ⶨ̼�ĺ�����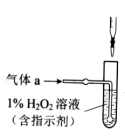
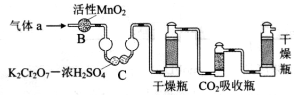
������aͨ��B��C��Ŀ����_____________________��
�ڼ��������̼������������Ӧ������������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����þ�������̼�ķ�Ӧ�Ʋ⣬��Ҳ���ڶ�����̼��ȼ�գ��ҹ���������Ϊ̼���ơ������ơ�̼�е����ֻ����֡�ij��ȤС������ڶ�����̼��ȼ�պ�IJ�����ж��ԺͶ���̽����
��1���������ΪNa2CO3��Na2O��C�Ļ������ʵ�鷽������֤���е�Na2CO3��Na2O���ڴ����д��ʵ�鲽�衢Ԥ������ͽ��ۡ�����֪����ʱBaCO3������Һ��pH=9.6��
��ѡ�Լ���������ϡ���ᡢBaCl2��Һ��Ba(OH)2��Һ������pH��ֽ����ȷ��0.1�����ձ����Թܡ��ιܡ���������������ɫ��
| ʵ����� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ�ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã�ȡ�ϲ���Һ���á� | �в��ܵĺ�ɫ���塣 |
| ����2��ȡ��������1��Һ���Թ��У� | |
| ����3�� | |
| �ζ� ���� | ������Һ �����/mL | ���ı��������� | |
| V1/mL | V2/mL | ||
| 1 | 25.00 | 15.02 | 4.97 |
| 2 | 25.00 | 14.98 | 5.03 |
| 3 | 25.00 | 13.21 | 6.75 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ��һ��ʵ�õġ����ĵ�ѧ�ƣ������е��������ⳣ�漰����ѧ֪ʶ�����ڸ����÷�չ��Ⱥ��������������ͬʱ��Ҳ���������Ӱ�졣�����й���������ȷ����
| A��������Ϊһ�ຬ�з����ȵ��л��������Ч�����ѵ���������������ƻ������㣬������Ա���ڻ����з������͵������Ʒ�Լ��ٷ�������ʹ���� |
| B������Ӧ��ԭ���ԭ���������˶��ֵ�أ���ɵ�ء�����ء����ܵ�صȡ�������ִ������Ϳ�ѧ�����з�������Ҫ���ã����Ͼɵ�ػ����̬�������ڽ������Σ�������ԷϾɵ�ر������������ |
| C��������ͳ�������ǿ������������ɱ��ˮ�е�ϸ��������ˮ����������ɱ������������Ϊ����ɱ������Ч��Ҫ�ȳ����ã��Ҳ�������ж����к����� |
| D��������������ˮ��������������������Σ����������Գմ����������������˴�������ˮ��ʹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
3��22��������ˮ�գ�����������ǡ�ˮ������(water cooperation)�������й�ˮ����������ȷ���ǣ� ��
| A����Ȼ���е�ˮ��ຬ���������� |
| B��ˮ��Ca2����Mg2��������ˮ�帻Ӫ���� |
| C���Ҷ������������ܻᵼ��ˮ��Ⱦ |
| D������ˮ�Ļ�ѧ���ʵ���С����ˮ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com