������ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ������������ �����������������ʣ�����ʵ�鲽����ͼһ��ʾ��
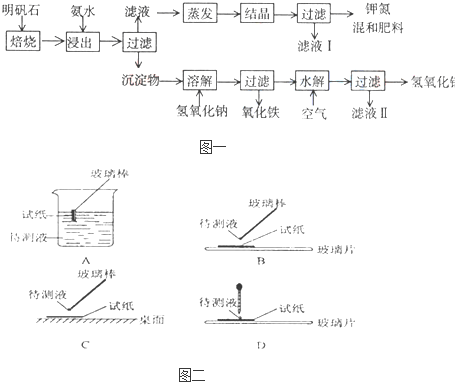
����ͼһʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500mLϡ��ˮ��ÿ������39.20g������ҪȡŨ��ˮ��ÿ ������250.28g����
78
78
mL���ù��Ϊ
100
100
mL��Ͳ��ȡ��
��2����ˮ������õ���Һ�����ϵ�����ˣ���Һ�г�K
+��SO
42-�⣬���д�����NH
4+������NH
4+�ķ�����
ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ����
ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ����
��
��3��д�����������������ʵĻ�ѧʽ
Al��OH��3��Al2O3��Fe2O3
Al��OH��3��Al2O3��Fe2O3
��
��4����ҺI�ijɷ���ˮ��
K2SO4����NH4��2SO4
K2SO4����NH4��2SO4
��
��5��Ϊ�ⶨ��Ϸ���K
2SO
4����NH
4��
2SO
4�мصĺ������������в��裺
�ٳ�ȡ�ص�������������ˮ����������
BaCl2��Ba��NO3��2
BaCl2��Ba��NO3��2
��Һ��������ɫ������
��
����
����
��
ϴ��
ϴ��
��
����
����
��������дʵ��������ƣ���
����ȴ�����أ�
��6��������Ϊmg�����������ʵ���Ϊnmol����������K
2SO
4�����ʵ���Ϊ��
mol���ú�m��n�Ĵ���ʽ��ʾ����
��ij��ɫ����Һ���ܺ����������ӣ�K
+��Al
3+��Fe
3+��Ba
2+��NO
3-��SO
42-��HCO
3-��Cl
-�ȣ�ȡ����Һ��������ʵ�飺
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬��������������������Ϊ���� ɫ��
��ȡ��Һ���������백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ��
��ȡ��Һ�����������Ȼ�����Һ������ɫ������
��ȡʵ�� �ܺ�ij�����Һ��������������Һ������ɫ�������ټ��������ϡ���ᣬ��������ʧ��
��ش��������⣺
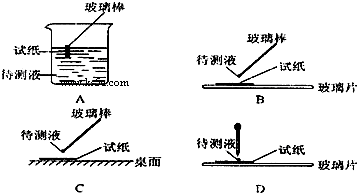
�� l ����ʵ�� ���У�ͼ����ʾ�IJ�������ȷ����
BD
BD
������ţ�
��2����������ʵ���ж�ԭ��Һ�п϶����ڵ�������
Al3+��NO3-��SO42-
Al3+��NO3-��SO42-
���϶������ڵ�������
Fe3+��Ba2+��HCO3-
Fe3+��Ba2+��HCO3-
��
��3��д����ڢ�����ʵ���йص����ӷ���ʽ��
��
3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O
3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O
��
��
Al3++3NH3?H2O�TAl��OH��3��+3NH4+
Al3++3NH3?H2O�TAl��OH��3��+3NH4+
��



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�

 ij��ɫ����Һ�п��ܺ�Mg2+��Al3+��Fe3+��Cu2+��NH4+��K+�еļ������ӣ�����������Ĺ�������ʱ������ɫ��ζ�����������ͬʱ���ɰ�ɫ����������Ĺ������Ƶ����������������֮��Ĺ�ϵ����ͼ��ʾ���Իش�
ij��ɫ����Һ�п��ܺ�Mg2+��Al3+��Fe3+��Cu2+��NH4+��K+�еļ������ӣ�����������Ĺ�������ʱ������ɫ��ζ�����������ͬʱ���ɰ�ɫ����������Ĺ������Ƶ����������������֮��Ĺ�ϵ����ͼ��ʾ���Իش�
