
¶žŃõ»ÆīŃ£ØTiO2£©¹ć·ŗÓ¦ÓĆÓŚø÷Ąą½į¹¹±ķĆęĶæĮĻ”¢Ö½ÕÅĶæ²ćµČ£¬¶žŃõ»ÆīŃ»¹æÉ×÷ĪŖÖʱøīѵ„ÖŹµÄŌĮĻ”£
¢ń£®¶žŃõ»ÆīŃæÉÓÉŅŌĻĀĮ½ÖÖ·½·ØÖʱø£ŗ
·½·Ø1£ŗTiCl4Ė®½āÉś³ÉTiO2”¤xH2O£¬¹żĀĖ”¢Ė®Ļ“³żČ„ĘäÖŠµÄCl££¬ŌŁŗęøÉ”¢±ŗÉÕ³żČ„Ė®·ÖµĆµ½½ŗĢåTiO2£¬“Ė·½·ØÖʱøµĆµ½µÄŹĒÄÉĆ׶žŃõ»ÆīŃ”£
£Ø1£©¢Ł TiCl4Ė®½āÉś³ÉTiO2”¤x H2OµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________ £»
¢Ś¼ģŃéTiO2”¤x H2OÖŠCl£ŹĒ·ń±»³ż¾»µÄ·½·ØŹĒ______________________________
·½·Ø2£ŗæÉÓĆŗ¬ÓŠFe2O3µÄīŃĢśæó£ØÖ÷ŅŖ³É·ÖĪŖFeTiO3£¬ĘäÖŠTiŌŖĖŲ»ÆŗĻ¼ŪĪŖ+4¼Ū£©ÖĘČ”£¬ĘäÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ
£Ø2£©īŃĢśæó·ŪÄ©ÖŠ¼ÓĮņĖį·“Ó¦µĆTiO2+µÄĄė×Ó·½³ĢŹ½ĪŖ
£Ø3£©ČÜŅŗ¼×ÖŠ¼ÓČėFeµÄ×÷ÓĆŹĒ
£Ø4£©ČōŅŖ¼ÓČČFeSO4£®7H2O¾§ĢåµĆĪŽĖ®ĮņĖįŃĒĢś£¬³ż¾Ę¾«µĘ”¢²£Į§°ōĶā£¬»¹ŅŖÓƵ½µÄĮ½ÖÖ¹čĖįŃĪÖŹŅĒĘ÷ŹĒ
¢ņ¶žŃõ»ÆīŃæÉÓĆÓŚÖĘČ”īѵ„ÖŹ£¬Éę¼°µ½µÄ²½ÖčČēĻĀĶ¼£ŗ
·“Ó¦¢ŚµÄ·½³ĢŹ½ŹĒ £¬øĆ·“Ó¦ŠčŅŖŌŚArĘų·ÕÖŠ½ųŠŠ£¬Ēė½āŹĶŌŅņ£ŗ _
¢ń£Ø1£©¢Ł TiCl4+ (x+2)H2O =TiO2”¤x H2O+ 4HCl
¢ŚČ”×īŗóŅ»“ĪµÄĻ“µÓĀĖŅŗ1”«2 mLÓŚŹŌ¹ÜÖŠ£¬ĻņĘäÖŠµĪ¼ÓĻõĖįĖį»ÆµÄAgNO3ČÜŅŗ£¬ČōĪŽ°×É«³Įµķ²śÉś£¬Ōņ±ķĆ÷³ĮµķŅŃĻ“µÓøɾ»”£
£Ø2£©FeTiO3+ 4H+ = TiO2 + +Fe2+ + 2H2O
£Ø3£©½«ČÜŅŗÖŠµÄFe3+»¹ŌĪŖFe2+
£Ø4£©ŪįŪö Ĺȿ½Ē
¢ņTiCl4£«2Mg£½2MgCl2£«Ti ·ĄÖ¹øßĪĀĻĀMg£ØTi£©ÓėæÕĘųÖŠµÄŃõĘų£Ø»ņCO2”¢N2£©×÷ÓĆ£©
½āĪöŹŌĢā·ÖĪö£ŗ£ŗ£Ø1£©¢ŁÉčTiCl4µÄĻµŹżĪŖ1£¬øł¾ŻŌŖĖŲŹŲŗć£¬TiO2?xH2OµÄĻµŹżĪŖ1£¬HClµÄĻµŹżĪŖ4£»ŌŁøł¾ŻOŌŖĖŲŹŲŗć£¬æÉÖŖH2OµÄĻµŹżĪŖ£Ø2+x£©£¬·½³ĢŹ½ĪŖTiCl4+£Øx+2£©H2O?TiO2?xH2O”ż+4HCl£¬¹Ź“š°øĪŖ£ŗTiCl4+£Øx+2£©H2O?TiO2?xH2O”ż+4HCl£»¢Ś³ĮµķĪüø½ČÜŅŗÖŠµÄCl-£¬øł¾Ż·¢Éś·“Ó¦£ŗCl-+Ag+ØTAgCl”ż£¬Č”ÉŁĮæĻ“µÓŅŗ£¬¼ģŃéČÜŅŗÖŠČܽāµÄĄė×ÓŹĒ·ń»¹“ęŌŚ£¬¹Ź“š°øĪŖ£ŗȔɣĮæĖ®Ļ“Ņŗ£¬µĪ¼ÓĻõĖįĖį»ÆµÄAgNO3ČÜŅŗ£¬²»²śÉś°×É«³Įµķ£¬ĖµĆ÷Cl-ŅŃ³ż¾»£»£Ø2£©øł¾ŻŃõ»Æ»¹ŌŌĄķ£¬īŃĢśæó·ŪÄ©ÖŠ¼ÓĮņĖį·“Ó¦µĆTiO2+µÄĄė×Ó·½³ĢŹ½ĪŖFe2O3+6H+=2Fe3++3H2O£¬FeTiO3+4H+=Fe2++TiO2++2H2O£»£Ø3£©øĆČÜŅŗÖŠŗ¬ÓŠĢśĄė×Ó£¬Ģś¾ßÓŠ»¹ŌŠŌ£¬Äܽ«ĢśĄė×ÓŃõ»ÆÉś³ÉŃĒĢśĄė×ÓĒŅ²»Ņż½ųŠĀµÄŌÓÖŹ£¬ĖłŅŌĢśµÄ×÷ÓĆŹĒ£ŗ½«Fe3+×Ŗ»ÆĪŖFe2+£¬¹Ź“š°øĪŖ£ŗ½«Fe3+×Ŗ»ÆĪŖFe2+£»£Ø4£©ČōŅŖ¼ÓČČFeSO4£®7H2O¾§ĢåµĆĪŽĖ®ĮņĖįŃĒĢś£¬³ż¾Ę¾«µĘ”¢²£Į§°ōĶā£¬»¹ŅŖÓƵ½µÄĮ½ÖÖ¹čĖįŃĪÖŹŅĒĘ÷ŹĒŪįŪöŗĶĹȿ½Ē”£ŌŚ800”ęĢõ¼žĻĀ£¬ĖÄĀČ»ÆīŃŗĶĆ¾·“Ӧɜ³ÉĀČ»ÆĆ¾ŗĶīŃ£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗTiCl4+2Mg=2MgCl2+Ti”£MgŹĒ»īĘĆ½šŹō£¬ÄÜÓėæÕĘųÖŠ¶ąÖÖĪļÖŹ·“Ó¦£¬Ņņ“ĖæÉµĆ³öArĘų×÷ÓĆĪŖ±£»¤Ęų£¬·ĄÖ¹MgŗĶæÕĘųÖŠĪļÖŹ·“Ó¦£¬¹Ź“š°øĪŖTiCl4+2Mg=2MgCl2+Ti£» ·ĄÖ¹øßĪĀĻĀMg£ØTi£©ÓėæÕĘųÖŠµÄO2£Ø»ņCO2”¢N2£©×÷ÓĆ”£
æ¼µć£ŗæ¼²éѧɜ¶Ō¹¤ŅÕĮ÷³ĢĄķ½ā”¢ŌĶĮĢāÄæ»ńČ”ŠÅĻ¢ÄÜĮ¦”¢Ńõ»Æ»¹Ō·“Ó¦¼°·½³ĢŹ½µÄŹéŠ“µČ£¬ÄѶČÖŠµČ£¬Ąķ½ā¹¤ŅÕĮ÷³ĢŹĒ¹Ų¼ü£¬ŠčŅŖѧɜ¾ßÓŠŌĶĮĢāÄæ»ńČ”ŠÅĻ¢ÄÜĮ¦ŗĶĮé»īŌĖÓĆ»ł“”ÖŖŹ¶·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāÄÜĮ¦”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŹĒŅ»ÖÖÖŲŅŖµÄ×ŌȻ׏Ō“£¬ŹĒČĖĄąĄµŅŌÉś“ę²»æÉȱɣµÄĪļÖŹ”£Ė®ÖŹÓÅĮÓÖ±½ÓÓ°ĻģČĖĢå½”æµ
£Ø1£©ŗ¬ÓŠ½Ļ¶ą______________µÄĖ®³ĘĪŖÓ²Ė®£¬Ó²Ė®¼ÓČČŗó²śÉś³ĮµķµÄĄė×Ó·½³ĢŹ½ĪŖ__________________________( Š“³öÉś³ÉŅ»ÖÖ³ĮµķĪļµÄ¼“æÉ) ”£
£Ø2£©ĻĀĶ¼ĪŖijŅūÓĆĖ®³§“ÓĢģČ»Ė®Öʱø“æ¾»Ė®(Č„Ąė×ÓĖ®)µÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼£ŗ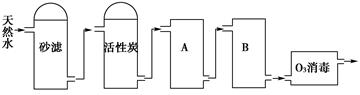
¢Ł»īŠŌĢæµÄ×÷ÓĆŹĒ__________________£»O3Ļū¶¾µÄÓŵćŹĒ________________”£
¢ŚA”¢BÖŠ·ÅÖƵÄĪļÖŹĆū³Ę·Ö±šŹĒ£ŗA__________________£»B_______________”£A”¢BÖŠ·ÅÖƵÄĪļÖŹŹĒ·ńæÉŅŌ»„»»?ĒėĖµĆ÷ŌŅņ________________________________________”£
£Ø3£©ĶعżŹ©¼ÓŅ»¶ØŃ¹Į¦Ź¹Ė®·Ö×ÓĶعż°ėĶøĤ¶ų½«“ó·Ö×Ó»ņĄė×Ó½ŲĮō, “Ó¶ų»ńµĆ“æ¾»Ė®µÄ·½·Ø³ĘĪŖ ”£µēÉųĪö·Ø¾»»ÆĖ®Ź±, Ź¹Ąė×ÓĶعż°ėĶøĤµÄĶʶÆĮ¦ŹĒ ”£
£Ø4£©¼ģŃéÕōĮóĖ®µÄ“æ¶ČŹ±, ×ī¼ņµ„Ņ׊ŠµÄ·½·ØŹĒ²ā¶ØĖ®µÄ ”£
£Ø5£©Ä³ ³Ē ŹŠ ÓĆ Ė® ÖŠc(Ca2+)ĪŖ1.0”Į10-3mol/L, c(Mg2+)ĪŖ5.0”Į10-4mol/L,c(HCO3_)ĪŖ8.0”Į10-4mol/L”£ČēÓĆŅ©¼ĮČķ»ÆøĆ1000L ,Ó¦¼ÓČėCa(OH) 2 g , Na2CO 3 __________ g ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)
LiPF6ŹĒļ®Ąė×Óµē³ŲÖŠ¹ć·ŗÓ¦ÓƵĵē½āÖŹ”£Ä³¹¤³§ÓĆLiF”¢PCl5ĪŖŌĮĻ£¬µĶĪĀ·“Ó¦ÖʱøLiPF6£¬ĘäĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ£ŗHClµÄ·ŠµćŹĒ£85.0 ”ę£¬HFµÄ·ŠµćŹĒ19.5 ”ę”£
£Ø1£©µŚ¢Ł²½·“Ó¦ÖŠĪŽĖ®HFµÄ×÷ÓĆŹĒ ”¢ ”£·“Ó¦Éč±ø²»ÄÜÓĆ²£Į§²ÄÖŹµÄŌŅņŹĒ (ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)”£ĪŽĖ®HFÓŠøÆŹ“ŠŌŗĶ¶¾ŠŌ£¬¹¤³§°²Č«ŹÖ²įĢįŹ¾£ŗČē¹ū²»Š”ŠÄ½«HFÕ“µ½Ę¤·ōÉĻ£¬æÉĮ¢¼“ÓĆ2%µÄ ČÜŅŗ³åĻ“”£
£Ø2£©øĆĮ÷³ĢŠčŌŚĪŽĖ®Ģõ¼žĻĀ½ųŠŠ£¬µŚ¢Ū²½·“Ó¦ÖŠPCl5¼«Ņ×Ė®½ā£¬Ęä²śĪļĪŖĮ½ÖÖĖį£¬Š“³öPCl5Ė®½āµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©µŚ¢Ü²½·ÖĄė²ÉÓƵķ½·ØŹĒ £»µŚ¢Ż²½·ÖĄėĪ²ĘųÖŠHF”¢HCl²ÉÓƵķ½·ØŹĒ ”£
£Ø4£©LiPF6²śĘ·ÖŠĶس£»ģÓŠÉŁĮæLiF”£Č”ѳʷwg”£²āµĆLiµÄĪļÖŹµÄĮæĪŖnmol£¬ŌņøĆѳʷ֊LiPF6µÄĪļÖŹµÄĮæĪŖ mol(ÓĆŗ¬ÓŠw”¢nµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Į×ĖįŃĪ¹ĒĖ®Äą¾ßÓŠĮ¼ŗƵÄÉśĪļĻąČŻŠŌŗĶÉśĪļ»īŠŌ”£Ņ½Ņ©¹¤ŅµĄūÓĆ¹²³ĮµķŌĄķ£¬ĶعżæŲÖĘCa/PĪļÖŹµÄĮæ±Čn(Ca)/n(P)]ÖʱøĻąÓ¦²śĘ·[Ca5(PO4)3OHŗĶCa3(PO4)2µÄn(Ca)/n(P)·Ö±šĪŖ1.67ŗĶ15]Į÷³ĢČēĻĀ£ŗ
(×¢£ŗCa5(PO4)3OHŗĶCa3(PO4)2ŗĶCaHPO4¾łÄŃČÜÓŚĖ®£»Ca(H2 PO4)2ČÜŅŗpH<7)
ĻĀ±ķĪŖn(Ca)/n(P)=1.5Ź±£¬²»Ķ¬pHÖµÖʵĆĀĖ±żµÄ²śĀŹŅŌ¼°·ÖĪö½į¹ū£ŗ
£Ø1£©Į÷³ĢÖŠĒæµ÷”°µĪ¼ÓĖŁ¶Č100mL/45minµÄ×÷ÓĆŹĒ ”£Į÷³ĢÖŠµ÷pHŃ”°±Ė®£¬²»Ń”ÉśŹÆ»Ņ»ņŹÆ»ŅČéµÄĄķÓÉŹĒ ”£
£Ø2£©“Ó±ķÖŠŹż¾Ż·ÖĪöÉś³ÉCa3(PO4)2Ź±£¬”°pH=x”±ÖŠµÄxµÄ×ī¼ŃȔֵĪŖ £¬ĀĖ±żµÄ»Æѧ³É·ÖCa5(PO4)3OH”¢Ca3(PO4)2ŗĶCaHPO4ŗĶ ”£
£Ø3£©ĖįŠŌĢõ¼žĻĀ²śĀŹĘ«µĶµÄŌŅņŹĒ ”£
£Ø4£©”°øßĪĀģŃÉÕ”±ĀĖ±ż£¬»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø5£©ČēĶ¼ŹĒÉś²śōĒ»łĮ×»ŅŹÆŹ±µĆµ½µÄŹµŃéĒśĻߣ¬ŅĄ¾ŻĶ¼ÉĻŠÅĻ¢¼ĘĖćĮ׵ijõŹ¼ÅضČĪŖ0.70mmol/L£¬pH=10.0Ģõ¼žĻĀ·“Ó¦Ē°10minÄŚĮ׵ijĮµķĖŁĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øßĢśĖįÄĘ£ØNa2FeO4£©¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬ŹĒŅ»ÖÖ±ČĀČĘųøüŗĆµÄ¾»Ė®Ļū¶¾¼Į”£¹¤ŅµÉĻæÉŅŌĶعż“ĪĀČĖįÄĘŃõ»Æ·ØÖʱøøßĢśĖįÄĘ£¬Éś²ś¹ż³ĢČēĻĀ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ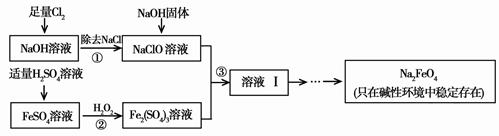
£Ø1£©ĀČĘų×÷¾»Ė®Ļū¶¾¼ĮŹĒŅņĪŖĖüČÜÓŚĖ®Éś³ÉĮĖ________£¬ĖüÓŠĒæµÄŃõ»ÆŠŌ£¬ÄÜɱ¾śĻū¶¾”£
£Ø2£©²½Öč¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø3£©“ÓČÜŅŗ¢ńÖŠ·ÖĄė³öNa2FeO4ŗ󣬻¹ÓŠø±²śĘ·Na2SO4”¢NaCl£¬Ōņ²½Öč¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø4£©½«Ņ»¶ØĮæµÄNa2FeO4Ķ¶Čėµ½pH²»Ķ¬µÄĪŪĖ®ÖŠ£ØĪŪĖ®ÖŠĘäÓą³É·Ö¾łĻąĶ¬£©£¬ČÜŅŗÖŠNa2FeO4ÅØ¶Č±ä»ÆČēĶ¼ĒśĻߢń”¢¢ņĖłŹ¾£¬ŹŌĶĘ²āĒśĻßII±ČĒśĻßI¶ŌÓ¦µÄĪŪĖ®pH________£ØĢī”°øß”±»ņ”°µĶ”±£©”£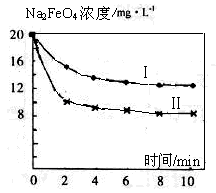
£Ø5£©Ķعż¼ĘĖćµĆÖŖNa2FeO4µÄĻū¶¾Š§ĀŹ£ØŅŌµ„Ī»ÖŹĮæµĆµ½µÄµē×ÓŹż±ķŹ¾£©Ō¼ŹĒĀČĘųµÄ_____±¶
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹¤ŅµÉĻÓĆ»ĘĶæóŅ±Į¶Ķ¼°¶ŌĀÆŌü×ŪŗĻĄūÓƵÄŅ»ÖÖ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
£Ø1£©Ņ±Į¶¹ż³ĢÖŠµĆµ½Cu2OŗĶCuµÄ»ģŗĻĪļ³ĘĪŖ”°ÅŻĶ”±£¬ĘäÓė½šŹōA1ŌŚøßĪĀĢõ¼žĻĀ»ģŗĻ·“Ó¦æɵƓÖĶ£¬·“Ó¦»Æѧ·½³ĢŹ½ĪŖ________”£“ÖĶ¾«Į¶Ź±Ó¦½«“ÖĶĮ¬½ÓŌŚÖ±Į÷µēŌ“µÄ____¼«£¬æÉŌŚ____¼«µĆµ½“æ¶Č½Ļøߵľ«Ķ”£
£Ø2£©“«Ķ³Į¶ĶµÄ·½·ØÖ÷ŅŖŹĒ»š·ØĮ¶Ķ£¬ĘäÖ÷ŅŖ·“Ó¦ĪŖ£ŗ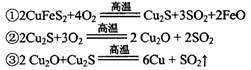
ĆæÉś³É1 mol Cu£¬¹²ĻūŗÄ____mol O2”£·“Ó¦¢ŪÖŠµÄŃõ»Æ¼ĮŹĒ____”£
£Ø3£©Į¶Ķ²śÉśµÄĀÆŌü(ŗ¬ )æÉÖʱøFe2O3”£øł¾ŻĮ÷³Ģ»Ų“šĻĀĮŠĪŹĢā£ŗ
)æÉÖʱøFe2O3”£øł¾ŻĮ÷³Ģ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł¼ÓČėŹŹĮæNaClOČÜŅŗµÄÄæµÄŹĒ_______ £ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©”£
¢Ś³żČ„Al3£«µÄĄė×Ó·½³ĢŹ½ŹĒ____”£
¢ŪŃ”ÓĆĢį¹©µÄŹŌ¼Į£¬Éč¼ĘŹµŃéŃéÖ¤ĀÆŌüÖŠŗ¬ÓŠFeO”£Ģį¹©µÄŹŌ¼ĮÓŠ£ŗĻ”ŃĪĖį”¢Ļ”ĮņĖį”¢KSCNČÜŅŗ”¢KMnO4ČÜŅŗ”¢NaOHČÜŅŗ”¢µāĖ®”£ĖłŃ”ŹŌ¼ĮŹĒ____”£ŹµŃéÉč¼Ę£ŗ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓĆĆ¢Ļõ£ØNa2SO4 ”ć10H2O£©Öʱø“æ¼ī”¢Ć÷·Æļ§[£ØNH4£©2Al£ØSO4£©2”ć12H2O]µÄÉś²ś¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ
£Ø1£©ČÜŅŗCÖŠµÄČÜÖŹÖ÷ŅŖŹĒ_____________
£Ø2£©Ć÷·Æļ§µÄČÜŅŗ³Ź_____ŠŌ£¬Ć÷·Æļ§æÉÓĆÓŚ¾»Ė®£¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ĘäŌĄķ______________________
£Ø3£©¹ż³Ģ¢ńÖŠµÄ·“Ó¦ĪĀ¶Č²»Äܳ¬¹ż40”ę£¬ĘäŌŅņŹĒ______________________________
£Ø4£©ŌĖÓĆ»ÆŃ§Ę½ŗāŌĄķ½āŹĶNa2SO4ÉŌ¹żĮæµÄŌŅņ
£Ø5£©Čō½«Al2£ØSO4£©3¼ÓČėµ½AÖŠ»į²śÉś“óĮæµÄ³ĮµķŗĶĘųĢå,µ¼ÖĀĆ÷·Æļ§µÄ²śĮæ½µµĶ,ĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶ²śÉśøĆĻÖĻóµÄŌŅņŹĒ___________________________________________
£Ø6£©ČÜŅŗEÖŠµÄČÜÖŹĄė×ÓĪŖ__________________
£Ø7£©ŅŃÖŖĆ÷·Æļ§µÄČܽā¶ČĖę×ÅĪĀ¶ČµÄÉżø߶ųŌö“󣬹ż³Ģ¢ņÖŠµĆµ½Ć÷·Æļ§µÄĻµĮŠŹµŃé²Ł×÷ŹĒ: ”¢ ”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ņ»ÖÖŗ¬ĀĮ”¢ļ®”¢īܵĵē×Ó·ĻĮĻÖŠ£¬ĀĮŅŌĀĮ²µÄŠĪŹ½“ęŌŚ£¬īÜŅŌCo3O4µÄŠĪŹ½“ęŌŚ£ØĪüø½ŌŚĀĮ²µÄµ„Ćę»ņĖ«Ćę£©£¬ļ®»ģŌÓÓŚĘäÖŠ”£“ÓøĆ·ĻĮĻÖŠ»ŲŹÕCo3O4µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
£Ø1£©ČÜŅŗAµÄČÜÖŹµÄÖ÷ŅŖ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©
£Ø2£©īÜŌüÖŠ¼ÓČėĻ”H2SO4Ėį»Æŗó£¬ŌŁ¼ÓČėNa2S2O3ČÜŅŗæÉŅŌ½ž³öīÜĄė×Ó£¬Ōņ½ž³öīÜĄė×ӵĥė×Ó·½³ĢŹ½ĪŖ£Ø²śĪļÖŠÖ»ÓŠŅ»ÖÖĖįøł£© ”£
£Ø3£©ŌŚŹµŃéŹŅÄ£Äā¹¤ŅµÉś²śŹ±£¬Ņ²æÉÓĆŃĪĖį½ž³öīÜĄė×Ó£¬µ«Źµ¼Ź¹¤ŅµÉś²śÖŠ²»ÓĆŃĪĖį£¬Ēė“Ó·“Ó¦ŌĄķ·ÖĪö²»ÓĆŃĪĖį½ž³öīÜĄė×ÓµÄÖ÷ŅŖŌŅņ£ŗ ”£
£Ø4£©¼ÓČėNaFµÄ·“Ó¦ĪŖ£ŗLi£«£«F£ LiF”ż£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK= ”£
LiF”ż£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK= ”£
£Ø5£©¼ÓČė30%Na2CO3ČÜŅŗµÄ×÷ÓĆŹĒ ”£
£Ø6£©ŌŚæÕĘųÖŠ¶ĶÉÕCoCO3Éś³ÉCo3O4µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŗĻ³ÉNH3ĖłŠčµÄH2æÉÓÉĆŗÓėH2O·“Ó¦ÖĘµĆ£¬ĘäÖŠÓŠŅ»²½·“Ó¦ĪŖCO(g)£«H2O(g) CO2(g)£«H2(g)””¦¤H<0”£ÓūĢįøßCO×Ŗ»ÆĀŹæɲÉÓƵķ½·ØæÉÄÜÓŠ£ŗ¢Ł½µµĶĪĀ¶Č£»¢ŚŌö“óŃ¹Ēæ””¢ŪŹ¹ÓĆ“ß»Æ¼Į””¢ÜŌö“óCOµÄÅØ¶Č£»¢ŻŌö“óĖ®ÕōĘųµÄÅØ¶Č£¬ĘäÖŠÕżČ·µÄ×éŗĻŹĒ(””””)”£
CO2(g)£«H2(g)””¦¤H<0”£ÓūĢįøßCO×Ŗ»ÆĀŹæɲÉÓƵķ½·ØæÉÄÜÓŠ£ŗ¢Ł½µµĶĪĀ¶Č£»¢ŚŌö“óŃ¹Ēæ””¢ŪŹ¹ÓĆ“ß»Æ¼Į””¢ÜŌö“óCOµÄÅØ¶Č£»¢ŻŌö“óĖ®ÕōĘųµÄÅØ¶Č£¬ĘäÖŠÕżČ·µÄ×éŗĻŹĒ(””””)”£
| A£®¢Ł¢Ś¢Ū | B£®¢Ü¢Ż | C£®¢Ł¢Ż | D£®¢Ż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com