
| A�� | ���ʵ���Ũ����ȵĢ�NH4Cl �ڣ�NH4��2SO4 ��NH4Al��SO4��2������Һ�У�c��NH4+�ɴ�С��˳��Ϊ���ۣ��ڣ��� | |
| B�� | 0.1mol•L-1CH3COOH��0.05mol•L-1NaOH��Һ�������ϣ�pH��7���� c��CH3COO-����c��Na+����cCH3COOH����c��H+�� | |
| C�� | 0.1 mol•L-1Na2CO3��0.05mol•L-1 NaHCO3��Һ�������ϣ� $\frac{3}{5}$c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| D�� | ��0.1 mol•L-1NaHC2O4��Һ��pH��7���У�c��OH-��+2c��C2O42-��=c��H+��+2c��H2C2O4�� |
���� A����NH4Cl �ڣ�NH4��2SO4 ��NH4Al��SO4��2���Ȳ�����ˮ�⣬��ڣ�NH4��2SO4 ��������NH4+����������NH4+��Ũ�ȴ��������������ʣ��٢���Һ��c��NH4+����������ˮ���Ӱ������жϣ�
B.0.1mol•L-1CH3COOH��0.05mol•L-1NaOH��Һ�������ϵõ������ʹ����ƵĻ����Һ��pH��7��Һ������˵���ǵ�����ڴ�������ӵ�ˮ�⣺
C.0.1 mol•L-1Na2CO3��0.05mol•L-1 NaHCO3��Һ����������Һ�д��������غ㣬0.15n��Na��=0.25n��C������3n��Na��=5n��C����
D����0.1 mol•L-1NaHC2O4��Һ��pH��7������Һ�����ԣ�HC2O4-�������ˮ�⣬��Һ�д��ڵ���غ�������غ���������
��� �⣺A����NH4Cl �ڣ�NH4��2SO4 ��NH4Al��SO4��2���Ȳ�����ˮ�⣬��ڣ�NH4��2SO4 ��������NH4+����������NH4+��Ũ�ȴ��������������ʣ��٢۶��������Т�NH4Cl��NH4+ˮ�⣬��NH4Al��SO4��2�������ӵ�ˮ���笠��������������ã�����Һ��c��NH4+���ڣ��ۣ��٣���A����
B.0.1mol•L-1CH3COOH��0.05mol•L-1NaOH��Һ�������ϵõ������ʹ����ƵĻ����Һ��pH��7��Һ������˵���ǵ�����ڴ�������ӵ�ˮ�⣬��Һ������Ũ�ȴ�СΪ��c��CH3COO-����c��Na+����c��CH3COOH����c��H+������B��ȷ��
C.0.1 mol•L-1Na2CO3��0.05mol•L-1 NaHCO3��Һ����������Һ�д��������غ㣬0.15n��Na��=0.25n��C������3n��Na��=5n��C����$\frac{3}{5}$c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3������C��ȷ��
D����0.1 mol•L-1NaHC2O4��Һ��pH��7������Һ�����ԣ����������غ��c��Na+��=c��HC2O4-��+c��H2C2O4��+c��C2O42-�������ݵ���غ��c��H+��+c��Na+��=c��HC2O4-��+2c��C2O42-��+c��OH-�������Ե�c��H+��+c��H2C2O4��=2c��C2O42-��+c��OH-������D����
��ѡBC��
���� ���⿼������ˮ���ԭ����Ӧ�á��������Һ�е���غ㡢�����غ������ע������ˮ���ʵ�ʺͰ���������ʵĵ��룬��Ӱ��ƽ���ƶ��ĽǶȷ�������Ũ�ȵĴ�С�Ƚ��ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ã�NH4��2Fe��SO4��2 ��Һ�����NaOH��Ӧ��Fe��OH��2��Fe2++2OH-=Fe��OH��2�� | |
| B�� | ��ϡ�������Һ��ȥ����Ʒ��������⣺Fe2O3+6H+=2Fe3++3H2O | |
| C�� | ��С�մ���Һ�м�������Ca��OH��2��Һ��Ӧ��Ca2++2OH-+2HCO3-=CaCO3��+2H2O+CO32- | |
| D�� | ��NaClO��Һ�е�������FeSO4��Һ����Ӧ�����ӷ���ʽΪ��2Fe2++ClO-+2H+�TCl-+2Fe3++H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
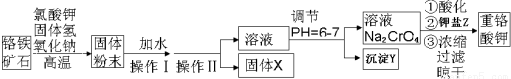
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
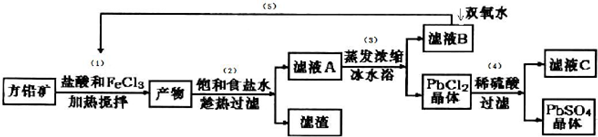

| ���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ |
| ����ǰŨ��/��mg•L-1�� | 0.100 | 29.8 | 0.12 | 0.087 |
| ������Ũ��/��mg•L-1�� | 0.004 | 22.6 | 0.04 | 0.053 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
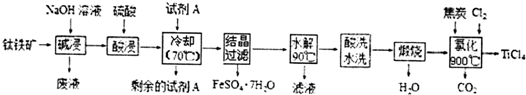
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
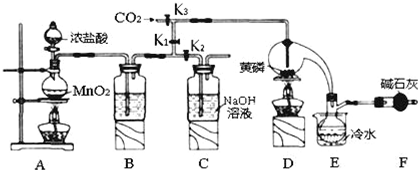
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
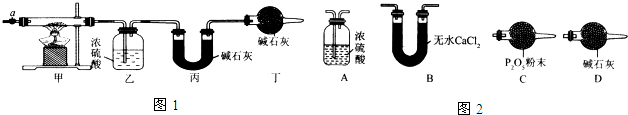
| ��װ�õ�����/g | ��װ�õ�����/g | |
| ����ǰ | 80.00 | 62.00 |
| ���Ⱥ� | 80.36 | 62.88 |
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 |
| ��ʼ������pH�� | 2.3 | 7.5 | 7.6 | 3.4 |
| ��ȫ������pH�� | 4.1 | 9.7 | 9.2 | 5.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �Թ�1 | �Թ�2 | |
| ʵ������ | ��ɫ�ܿ� | ��ɫ���� |
| ��ɫʱ��/s | 4�� | 31�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com