
���ƶ����л�����Ļ�ѧʽ��
(1)X��Y��Ԫ�����γ�XY2�ͻ����XY2�й���38�����ӣ���XY2�����ӻ�����仯ѧʽ��________��________����XY2�ǹ��ۻ�����仯ѧʽ��________��
(2)��3�����ڣ�A��B��Ԫ�ص�ԭ������֮��Ϊ4��������ɵĻ�����AB�Ļ�ѧʽΪ________��
(3)1molij���ʺ��в�ͬ���ڵ�����Ԫ�ظ�1mol����˵������Ϊ20mol�������ʵĻ�ѧʽΪ________��
(4)ij�ǽ���X����������ϼ�Ϊ��m��������������������Ӧ��ˮ�����γɵ�������b����ԭ�ӣ���������Ļ�ѧʽ��________��
(5)XԪ�ص�������ۺ��۾���ֵ֮��Ϊ6��YԪ�غ�XԪ��ԭ�ӵĴ�����Ӳ��϶���8�����ӣ�X��Y�γɵĻ�������ˮ��Һ���ܵ�������Ӳ�ṹ��ͬ�����ӣ���X��Y���γɵĻ�������________��
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| CH4 | SiH4 | NH3 | PH3 | |
| �е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
| �ֽ��¶ȣ�K�� | 873 | 773 | 1073 | 713.2 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 0.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һģ ���ͣ��ʴ���
| CH4 | SiH4 | NH3 | PH3 | |
| �е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
| �ֽ��¶ȣ�K�� | 873 | 773 | 1073 | 713.2 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 0.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��㶫ʡ�����и߿���ѧһģ�Ծ��������棩 ���ͣ������
| CH4 | SiH4 | NH3 | PH3 | |
| �е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
| �ֽ��¶ȣ�K�� | 873 | 773 | 1073 | 713.2 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 0.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����߿����� ���ͣ��ƶ���
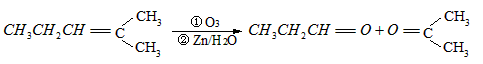
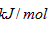 ����ͪ��ȼ����Ϊ1789
����ͪ��ȼ����Ϊ1789 ����д����ȩȼ�յ��Ȼ�ѧ����ʽ_____________ ��
����д����ȩȼ�յ��Ȼ�ѧ����ʽ_____________ ��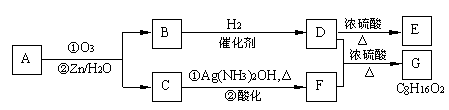
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ƶ����л�����Ļ�ѧʽ��
��1����3�����ڣ�A��B��Ԫ�ص�ԭ������֮��Ϊ4��������ɵĻ�����AB�Ļ�ѧʽΪ_____ ___________��
��2��1molij���ʺ��в�ͬ���ڵ�����Ԫ�ظ�1mol����˵������Ϊ20mol�������ʵĻ� ѧʽΪ_______________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com