
(9��)ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪Ũ�ȵ�����������Һʱ��ѡ�������ָʾ����
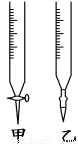
����д���пհף�
��1����ʽ�ζ���������ˮϴ��������Ӧ�ý��еIJ�����_________��
��2���ñ�������Һ�ζ�ʱ��Ӧ����������Һע��________(��ס����ҡ�)�С�
��3���ñ�������ζ����������������Һʱ����������ʽ�ζ��ܵĻ�������������ҡ����ƿ���۾�ע��_______________________________________________________��
��4��ֱ�������һ���������Һ��ɫ��________ɫ��Ϊ________ɫ������30 s�ڲ��䣬��Ϊ�յ㡣
��5�����в����п���ʹ��������������ҺŨ��ƫ�����________��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��װ������ᡣ
B����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ��
C���ζ�ǰʢ������������Һ����ƿ������ˮϴ����δ���
D����ȡ���������ʼʱ���Ӷ������ζ��������Ӷ�����
(9��)��1���ñ�������Һ��ϴ(2��) ����2�� �ף�1�֣���
��3����ƿ����Һ����ɫ�仯(2��)
��4���ɻƣ�1�֣�ɫ��Ϊ��ɫ��1�֣� ����4��AB(2��)��
��������
�����������1����ʽ�ζ���������ˮϴ����Ϊ��ϴȥ����ˮ��Ӧ�ñ�������Һ��ϴ2-3�Σ���2���ñ�������Һ�ζ�ʱ��Ӧ����������Һע����ʽ�ζ����У���3���ζ�ʱ���۾�ע����ƿ����Һ����ɫ�仯������Һ�ɻ�ɫ��Ϊ��ɫ�ﵽ�ζ��յ㣻��4�������ڼ��������³ʻ�ɫ�������������³ʺ�ɫ������ɫΪ��ɫ����ɫ��ΧpHΪ3��1-4��4����5��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��װ������ᣬ�����ᱻϡ�ͣ��������ƫ��������������Ũ��ƫ�ߣ�B����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ������������Һ�����ƫ��������ƫ�ߣ�C���ζ�ǰʢ������������Һ����ƿ������ˮϴ����δ�����Ӱ��ζ������D����ȡ���������ʼʱ���Ӷ������ζ��������Ӷ������������������ƫС���ⶨ���ƫ�ͣ���ѡAB��
���㣺����к͵ζ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ӱ�ʡ�߶�12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���з����к�������̼ԭ�ӵ���
A��CF2Cl2
B��CH3CH2OH
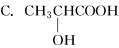
D��CH2===CH��COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶���ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���й��ڵ������Һ��������Ŀ���ж���ȷ����
A��0.1L 3.0mol/L��NH4NO3��Һ�к��е�NH4+����ĿΪ0.3NA
B��������������ʵ���Ũ�ȵ�NaCl��KCl��Һ�У�������������Ŀ֮�;�Ϊ2NA
C��0.1mol/L��NaHSO4��Һ�У������ӵ���Ŀ֮��Ϊ0.2NA
D��25��ʱ��pH=13��1.0LBa(OH)2��Һ�к��е�OH-��ĿΪ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ��ʡУ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�������һ���ܴ����������
A������ɫ��Һ�У�NH4+��Fe3+��SO42-��NO3-
B���ں�����Fe3+����Һ�У�NH4+��Na+��I-��OH-
C����ǿ����Һ�У�Na+��K+��Cl-��CO32-
D����pH=1����Һ�У�K+��Fe2+��Cl-��CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ��ʡУ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
O2��SO2��SO3���ߵ�������Ϊ2��4��5ʱ�����ǵ����ʵ���֮��Ϊ
A��2��4��5 B��1��2��3 C��1��1��1 D��2��2��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ�����и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���1mol��CuSO4��5H2O(s)����ˮ��ʹ��Һ�¶Ƚ��ͣ���ЧӦΪ��H1��1mol��CuSO4(s)����ˮ��ʹ��Һ�¶����ߣ���ЧӦΪ��H2��CuSO4��5H2O���ȷֽ�Ļ�ѧ����ʽΪ��CuSO4��5H2O(s)  CuSO4(s)+5H2O(l)����ЧӦΪ��H3���������ж���ȷ����
CuSO4(s)+5H2O(l)����ЧӦΪ��H3���������ж���ȷ����
A����H2����H3 B����H1����H3
C����H1+��H3 =��H2 D����H1+��H2 ����H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ�����и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�ܱ���ƿ�У���25 ��ʱ��������ƽ�⣺2NO2(g)  N2O4(g) ��H<0������ƿ����100 ���ˮ�У������м��������в���ı����
N2O4(g) ��H<0������ƿ����100 ���ˮ�У������м��������в���ı����
����ɫ ��ƽ����Է������� ������ ��ѹǿ ���ܶ�
A���ٺ͢� B���ں͢� C���ܺ͢� D���ۺ͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ������
��6�֣����ף�P���Ͱ��ף�P4����Ϊ��ͬ�������塣��֪��
P4 ( ��s ) �� 5O2 ( g ) �� P4O10 ( s ) ��H = -2983.2 kJ/mol
P (�� s ) �� 5/4O2 ( g ) �� 1/4P4O10 ( s ) ��H = -738.5 kJ/mol
д������ת��Ϊ�����Ȼ�ѧ����ʽ ,�ɴ˿�֪�����ױȰ��� ������ȶ������ȶ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��Ħ�����߸�����������֮һ
B�������ӵ���������6.02��1023 mol-1
C��1 mol���������2 g
D��1mol�����������NA��������ӵ�����֮�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com