
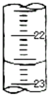
 ʵ�������к͵ζ����ⶨ�����ռ���Һ��Ũ�ȣ��Ը���ʵ��ش�
ʵ�������к͵ζ����ⶨ�����ռ���Һ��Ũ�ȣ��Ը���ʵ��ش�| �ζ����� | ����Һ���/mL | ���������/mL | |
| �ζ�ǰ����/mL | �ζ������/mL | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
| ������ | 10.00 | 4.20 | 25.70 |
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |
| 0.2000mol?L-1��0.02L |
| 0.01L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HClO�Ľṹʽ��H-Cl-O |
| B�������Ͷ���������������������������� |
C��������ӵı���ģ����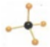 |
D�����ĵ���ʽ��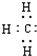 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | D | |
| �о�Ŀ�� | ѹǿ�Է�Ӧ��Ӱ�� | �¶ȶԷ�Ӧ��Ӱ�� | ����O2Ũ�ȶԷ�Ӧ��Ӱ�� | Ũ�ȶ�ƽ�ⳣ����Ӱ�� |
| ͼʾ | 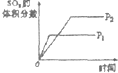 |  | 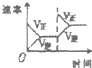 |  |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ΪNA��CO��C2H4����������ԼΪ22.4 L������Ϊ28g |
| B�������£�1L0.1mol��L-1��NH4NO3��Һ��NH4+��NO3-����Ϊ0.2NA |
| C����״���£�4.48 L��ˮ��D20�����е�������Ϊ2NA |
| D��1 mol����-CH3������������Ϊ9NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
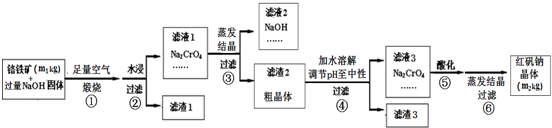
| �ᾧ�¶�/�� | Na2CrO4�־��и����ʺ���/w% | |||
| Na2CrO4?4H2O | NaOH | Na[Al��OH��4] | Na2SiO3 | |
| 30 | 52.45 | 29.79 | 8.69 | 12.21 |
| 40 | 68.81 | 20.49 | 8.46 | 10.84 |
| 50 | 60.26 | 27.96 | 10.36 | 9.32 |
| 60 | 50.74 | 29.66 | 10.40 | 12.25 |
| 70 | 46.77 | 33.06 | 8.10 | 6.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
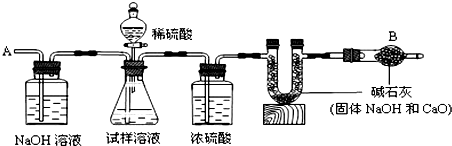
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
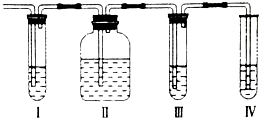 ��ʵ��������ȡ����ϩ�г����������Ķ�������ij��ѧ��ȤС���������ͼ��ʾ��ʵ��װ����ȷ����������������Ƿ���SO2��C2H4����ش��������⣺
��ʵ��������ȡ����ϩ�г����������Ķ�������ij��ѧ��ȤС���������ͼ��ʾ��ʵ��װ����ȷ����������������Ƿ���SO2��C2H4����ش��������⣺| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
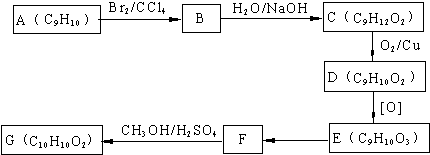
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com