
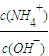 ��ֵ______�������С�����䡱��
��ֵ______�������С�����䡱�� NH4++OH-���淴Ӧ�����ƶ���c��OH-����С��
NH4++OH-���淴Ӧ�����ƶ���c��OH-����С�� NH4++OH-���淴Ӧ�����ƶ���c��OH-����С����c��NH4+������������Һ��
NH4++OH-���淴Ӧ�����ƶ���c��OH-����С����c��NH4+������������Һ��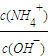 ��ֵ���ʴ�Ϊ������
��ֵ���ʴ�Ϊ������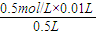 =0.01mol?L-1����Һ�е�c��H+��=10-2mol?L-1����Һ�е�c��OH-��=10-12 mol?L-1����Һ�е�OH-�͵���ˮ�������OH-��������Һ����ˮ�������c��H+��=c��OH-��=10-12mol?L-1��
=0.01mol?L-1����Һ�е�c��H+��=10-2mol?L-1����Һ�е�c��OH-��=10-12 mol?L-1����Һ�е�OH-�͵���ˮ�������OH-��������Һ����ˮ�������c��H+��=c��OH-��=10-12mol?L-1��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(NH4+) | c(OH-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������Ũ�Ⱦ�Ϊ0.1mol?L-1��������Һ����̼������Һ����̼��������Һ��������ܰ�ˮ���������ĿҪ��ش��������⣮
��������Ũ�Ⱦ�Ϊ0.1mol?L-1��������Һ����̼������Һ����̼��������Һ��������ܰ�ˮ���������ĿҪ��ش��������⣮| c(OH-) | c(NH3?H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(OH-) | c(NH3?H2O) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com