

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ������Һ25.00ml����������0.1%��A��Һ��ָʾ����������Ũ��Ϊ0.002000mol/L�������������Һ�ζ�������¼���������������Һ�������
������Һ25.00ml����������0.1%��A��Һ��ָʾ����������Ũ��Ϊ0.002000mol/L�������������Һ�ζ�������¼���������������Һ�������| ��� | �ζ�ǰ/mL | �ζ���/mL |
| �� | 1.35 | 19.40 |
| �� | 1.05 | 19.00 |
| �� | 1.58 | 20. 91 91 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A���٢ڢ� | B���ڢ� | C���٢ڢܢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ϩ���ֱ��ȼ���۲�������ɫ���Ƿ��к��� |
| B������ƾ��е�����ˮ����ƾ��м���������ʯ�� |
| C���Ʊ������飨C2H5Cl����������������Ļ�������ڹ��������·�Ӧ |
| D����֤ijRX�ǵ���飬��RX���ռ�ˮ��Һ��ϼ��Ⱥ���Һ��ȴ���ټ�����������Һ�۲��Ƿ���ֻ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Bƫ�͡�C��Ӱ�죩
Bƫ�͡�C��Ӱ�죩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
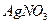 ��
��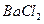 ��
��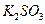 ��
��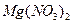 ������Һ������������֮������Ӧ�����Լ����� �� ��
������Һ������������֮������Ӧ�����Լ����� �� ��| A�����ᡢ���� | B�� ���ᡢ����������Һ ���ᡢ����������Һ |
| C����ˮ������ | D����ˮ������������Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com