
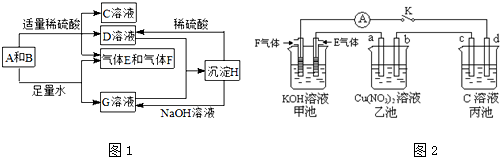
| ||
| ����ʯ |
| ||
| ����ʯ |
 ��
�� ��
�� Al��OH��3+3H+��
Al��OH��3+3H+�� Al��OH��3+3H+��
Al��OH��3+3H+��| 36g |
| 18g/mol |
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������ڢ��壬p�� |
| B���������ڢ�B�壬ds�� |
| C���������ڢ��壬d�� |
| D���������ڢ�B�壬f�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������COCl2�� |
| B�����Ȼ��ף�PCl5�� |
| C����������CF2Cl2�� |
| D����������BF3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
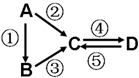 A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ��
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
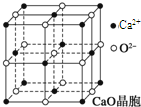 ���������з�Ӧ�ϳ������谷��CaO+3C
���������з�Ӧ�ϳ������谷��CaO+3C
| ||
| ||
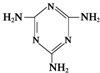 �׳ơ����������������������谷����������
�׳ơ����������������������谷����������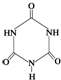 �����������������谷�����֮��ͨ��
�����������������谷�����֮��ͨ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4O��C2H6Oһ����Ϊͬϵ�� |
| B��1mol C6H6�������к���3mol̼̼˫�� |
| C����Ũ�����뵰���ʵ���ɫ��Ӧ���ֵ����� |
| D�������������м���NaOH ��Һ������Һ�����ȥ���������е��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����NaHCO3��Һ���У�C��CO32-��+C��OH-��=C��H+��+C��H2CO3�� |
| B��FeCl3��Һ�д��ڣ�Fe3++3H2O?Fe��OH��3+3H+ ��ˮ��ƽ�⣬������Һ�������HCl��NaOH��Һ������ʹ��ƽ�������ƶ� |
| C��������������ȡʵ���У��Լ������˳���ǣ���ˮ�Ҵ���Ũ����������� |
| D��ʵ������ȡ�����ԭ���ǣ���ˮ�����ƺͼ�ʯ�ҹ��ȣ����м�ʯ�ҵijɷ���NaOH��CaO |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com