
(1)����п�ǽ���п��(��Ҫ�ɷ���ZnS)ͨ����ѡ����ʹ��ת��Ϊ����п���ٰ�����п�ͽ�̿��ϣ��ڹķ�¯�м��ȵ�1 100��1 300 �棬ʹп���������
��д������п����Ҫ��Ӧ��
���շ�Ӧ��_________________________________________��
�ķ�¯�п��ܷ����ķ�Ӧ��___________________(��дһ��)
�ڴӱ��������ͳ������ԭ�ϽǶȿ���δ��������ò�����������_____________________________________________________________________________��
(2)��ҵ��ұ�������ǵ��������
��ұ�����ĵ����е��������������Ͼ���ʯ�����ƺ�ú�ĸ����Ʒ________(����������)��
�����������۵�ܸߣ�������ұ����Ҫ�������ʯ(Na3AlF6)����������________________________________________________��
�۹�ҵ��ұ����ʱ�õ�ԭ����Al2O3��������AlCl3����ԭ����
______________________________________________________��
(3)��ҵ�ϡ������Ƽ������Ҫ��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
���е�CO2��Դ��______________��
(4)̼������Ʋ�����ԭ��֮һ����ҵ���Ʋ������ڲ�����¯�н��У����з�Ӧ֮һΪCaCO3��SiO2 CaSiO3��CO2�����������������£���1 000a g CaCO3��60a g SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ________________(�ú�a�Ĵ���ʽ��ʾ)��
CaSiO3��CO2�����������������£���1 000a g CaCO3��60a g SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ________________(�ú�a�Ĵ���ʽ��ʾ)��
 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͨ��Ũ��ˮ�У��������������з�Ӧ��3Cl2��8NH3·H2O= ==6NH4Cl��N2��8H2O���ڱ�״���£���1.12 L Cl2��N2�Ļ������(90% Cl2��10% N2����Ϊ�������)ͨ��Ũ��ˮ��ʵ�����ݳ��������Ϊ0.672 L(������50% Cl2��
==6NH4Cl��N2��8H2O���ڱ�״���£���1.12 L Cl2��N2�Ļ������(90% Cl2��10% N2����Ϊ�������)ͨ��Ũ��ˮ��ʵ�����ݳ��������Ϊ0.672 L(������50% Cl2�� 50% N2)���˷�Ӧ�б�������NH3������Ϊ (����)
50% N2)���˷�Ӧ�б�������NH3������Ϊ (����)
A��3.4 g B��0.34 g
C��1.36 g D��4.48 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ɻ�ͭ��(��Ҫ�ɷ���CuFeS2) ���ƾ�ͭ�Ĺ�������ʾ��ͼ���£�
���ƾ�ͭ�Ĺ�������ʾ��ͼ���£�
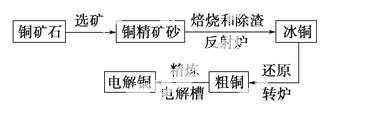
(1)�ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1 000 �����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ�����Ҳ���Fe������ת��Ϊ�ͼ�������ù�����������Ҫ��Ӧ�Ļ�ѧ����ʽ�ֱ���____________________��__________________________������¯������ ¯������Ҫ�ɷ���________��
¯������Ҫ�ɷ���________��
(2)��ͭ(Cu2S��FeS�����ۺ϶���)��Cu��Ϊ20%��50%��ת¯�У�����ͭ���ۼ�(ʯӢɰ)��1 200 �����Ҵ���������д�������ͭ�е�Cu2S��������Cu2O�����ɵ�Cu2O��Cu2S��Ӧ�����ɺ�Cu��ԼΪ98.5%�Ĵ�ͭ���ù��̷�����Ӧ�Ļ�ѧ����ʽ�ֱ���____________��
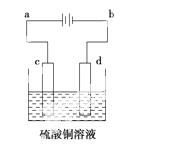
(3)��ͭ�ĵ�⾫������ͼ��ʾ���ڴ�ͭ�ĵ������У���ͭ��Ӧ��ͼ�е缫________(��ͼ�е���ĸ)���ڵ缫d�Ϸ����ĵ缫��ӦʽΪ____________________________������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ����ش��������⣺
(1)Ŀǰ�����¡��������У������ڡ���ˮ����������___________________________
(�����)��
��������Ĥ���뷨(������������)�����䶳���������ӽ�����
(2)�� ˮɹ�����õĻ�ѧԭ����____________________________________________��
ˮɹ�����õĻ�ѧԭ����____________________________________________��
����ʳ�ξ�����ĸҺ�к���____________���������롢�ᴿ������_________��
(3)��ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ����Ӧ�� ���ӷ���ʽΪ________________________________________________________________________��
���ӷ���ʽΪ________________________________________________________________________��
������õ�ʳ��ˮ��Ҫ���ƣ���ԭ����_____________________________________
________________________________________________________________________��
����ʱ�����Լ������ᡢBaCl2��Һ��NaOH��Һ��Na2CO3��Һ�������Լ�ʱ��Na2CO3��Һ�����ڼ���__________________________________________________________֮����롣
(4)���������������һ�������ȼҵ��Ʒ���Ȼ���ѭ����������������ķ���ͬʱ���ն�������ķ������÷������������£�
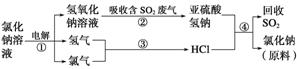
д����Ӧ�ڡ��ܵĻ�ѧ����ʽ��_____________________________________________��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ���ҵ�����Ṥҵ���������� ������ͼ��ʾ ��
��

�ϳ����ͽӴ����еķ�Ӧ�ֱ�Ϊ
N2(g)��3H2(g) 2NH3(g)����H��0��
2SO2(g)��O2(g) 2SO3(g)����H��0��
(1)д���������豸�����ƣ�B________��X________��
(2)����ϳ����ͽӴ����е����嶼Ҫ�����ȴ�������������ȴ���������___________________��
(3)����ѭ�����������ԭ�ϵ������ʣ����������У�����ѭ����������________(�����)��
�����Ṥҵ���ںϳɰ���ҵ�������Ṥҵ
(4)��ҵ�ϳ���98.3%��Ũ��������SO3������ϡ�����ˮ��ԭ����_______________��
(5)��ҵ�����г��ð����ᷨ����β�������Դﵽ�� ����Ⱦ��
����Ⱦ�� �������õ�Ŀ�ġ����Ṥҵβ���е�SO2���������Եõ�һ�ֻ��ʣ��÷��ϵĻ�ѧʽ��___________________��
�������õ�Ŀ�ġ����Ṥҵβ���е�SO2���������Եõ�һ�ֻ��ʣ��÷��ϵĻ�ѧʽ��___________________��
(6)���ݻ�ѧ ƽ���ƶ�ԭ���������������ʩ��������________(�����)��
ƽ���ƶ�ԭ���������������ʩ��������________(�����)��
�ٺϳɰ���ҵ�ڸ�ѹ�½���
�ںϳɰ���ҵ�����Ṥҵ��ʹ�ô���
�ۼ�ʱ����Һ��������
�����Ṥҵ�У��������¯����Ҫ�й�������
�ݺϳɰ���ҵ�����Ṥҵ���������˵��¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯ��Ҫ������ơ�Ca3(PO4)2·H2O������ʯ��Ca5(OH)(PO4)3������ʽ���ڡ�ͼ(a)ΪĿǰ��������ʯ���õĴ������������ʪ��������ָ��ʯ�ù�������ֽ��Ʊ����ᡣͼ(b)���ȷ�������������������ʯ�Ƶ��������̡�
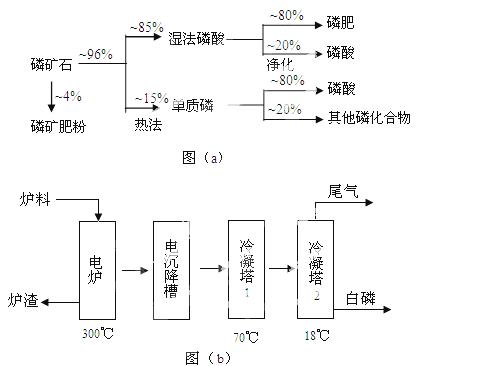
�������ʵ�����������£�
| �۵�/�� | �е�/�� | ��ע | |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ�����л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
�ش��������⣺
��1����������ʯ����Ҫ����;�����������ϣ�Լռ��ʯʹ������ ℅��
��2������ʯΪԭ�ϣ�ʪ�����������Ca5F(PO4)3��Ӧ�Ļ�ѧ����ʽΪ��  ������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85℅����Ʒ
������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85℅����Ʒ ���� �֡�
���� �֡�
��3����ͼ(b)��ʾ���ȷ���������ĵ�һ���ǽ��������衢������̿����ʯ��ϣ����·�Ӧ���ɰ��ס�¯������Ҫ�ɷ��ǣ� (�ѧʽ)������1����Ҫ�������ǣ� ������2����Ҫ�������ǣ�
��4��β������Ҫ���� ������������PH3��H2S��HF�ȣ���β ����ͨ�봿����Һ��
����ͨ�봿����Һ�� �ɳ�ȥ
�ɳ�ȥ 
��ͨ�����������Һ���ɳ�ȥ (���ѧʽ)
��5�������ʪ�����ᣬ�ȷ����Ṥ�ո��ӣ��ܺĸߣ����ŵ��ǣ�  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���绷�����˽���ȫ���ֹʹ��������������ˮ����������������ø�Ч����ɫ���������������ȡ�����������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ���������ϡ�ͣ�ͬʱ��Ҫ������ա�����ȡ�ʵ�����Ե�ⷨ�Ʊ�ClO2���������£�
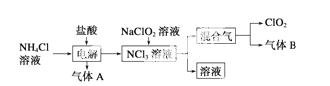
(1)ClO2������ԭ��________(��ǡ����ǡ�)������8���ӽṹ����ͼ��ʾ��ⷨ�ƵõIJ�������������B��ʹʯ����Һ����ɫ����ȥ���������ѡ��________��
A������ʳ��ˮ B����ʯ��
C��Ũ���� D������ˮ
(2)�ȶ��Զ���������Ϊ�ƹ�������ȶ����������Ͳ�Ʒ������˵����ȷ����________��
A���������ȿɹ㷺���ڹ�ҵ������ˮ����
B��Ӧ����ʳƷ��ҵ������Ч���ӳ�ʳƷ������
C���ȶ��Զ������ȵij��ִ�������˶������ȵ�ʹ�÷�Χ
D���ڹ������ͳ�Ʒ�������ڣ�Ҫ��ͨ��װ�úͼ�⼰����װ��
(3)ŷ������Ҫ��������������Ũ�����Ʊ�����ѧ��Ӧ����ʽΪ________________________________________________________________��
ȱ����Ҫ�Dz��ʵ͡���Ʒ���Է��룬��������Ⱦ������
(4)�ҹ��㷺���þ��������ϡ�͵������������������(NaClO2)��Ӧ�Ʊ�����ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
�˷����ŷ�������ŵ���______________________________________________��
(5)��ѧ�����о�����һ���µ��Ʊ����������������ữ�IJ���(H2C2O4)��Һ��ԭ�����ƣ���ѧ��Ӧ����ʽΪ_________________________________________________________
________________________________________________________________________��
�˷���������������桢����İ�ȫ�ԣ�ԭ����________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ�� b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӡ��ش��������⣺
b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӡ��ش��������⣺
��1��b��c��d�е�һ������������ ����Ԫ�ط��ţ���e�ļ۲���ӹ��ʾ��ͼΪ ��
��2��a������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪ �������� �Ⱥ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ������� ���ѧʽ��д���֣���
�Ⱥ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ������� ���ѧʽ��д���֣���
��3����ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������ ������������ṹ������ �����ѧʽ��
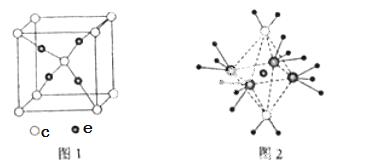
��4��e��c�γɵ�һ�����ӻ�����ľ���ṹ��ͼ1����e���ӵĵ��Ϊ ��
��5����5��Ԫ���γ� ��һ��1:1�����ӻ������У������ӳ�������ṹ�������ӳ����������İ�����ṹ����ͼ2��ʾ�����û�������������Ϊ ���������д��ڵĻ�ѧ�������� ���û��������ʱ����ʧȥ�������
��һ��1:1�����ӻ������У������ӳ�������ṹ�������ӳ����������İ�����ṹ����ͼ2��ʾ�����û�������������Ϊ ���������д��ڵĻ�ѧ�������� ���û��������ʱ����ʧȥ�������  ���ж������� ��
��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���Ħ��������˵����ȷ����(����)
A��ˮ��Ħ��������18 g
B��2 molˮ��Ħ��������1 molˮĦ��������2��
C���κ����ʵ�Ħ������������������Է������������ԭ������
D��ˮ��Ħ������������Ħ��������9��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com