

£Ø1£©AÖŠŹŌ¼ĮĪŖ______________”£
£Ø2£©ŹµŃéĒ°£¬ĻČ½«ĀĮĆ¾ŗĻ½šŌŚĻ”ĖįÖŠ½žÅŻĘ¬æĢ£¬ĘäÄæµÄŹĒ____________________________”£
£Ø3£©¼ģ²éĘųĆÜŠŌ£¬½«Ņ©Ę·ŗĶĖ®×°Čėø÷ŅĒĘ÷ÖŠ£¬Į¬½ÓŗĆ×°ÖĆŗ󣬊č½ųŠŠµÄ²Ł×÷»¹ÓŠ£ŗ¢Ł¼ĒĀ¼CµÄŅŗĆęĪ»ÖĆ£»¢Ś½«BÖŠŹ£Óą¹ĢĢå¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬³ĘÖŲ£»¢Ū“żBÖŠ²»ŌŁÓŠĘųĢå²śÉś²¢»Öø“ÖĮŹŅĪĀŗ󣬼ĒĀ¼CµÄŅŗĆęĪ»ÖĆ£»¢ÜÓÉAĻņBÖŠµĪ¼Ó×ćĮæŹŌ¼Į£»¢Ż¼ģ²éĘųĆÜŠŌ”£ÉĻŹö²Ł×÷µÄĖ³ŠņŹĒ___________£»£ØĢīŠņŗÅ£©¼ĒĀ¼CµÄŅŗĆęĪ»ÖĆŹ±£¬³żŹÓĻßĘ½ŹÓĶā£¬»¹Ó¦_______________”£
£Ø4£©BÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________________________”£
£Ø5£©ČōŹµŃéÓĆĀĮĆ¾ŗĻ½šµÄÖŹĮæĪŖa g£¬²āµĆĒāĘųĢå»żĪŖb mL£ØŅŃ»»ĖćĪŖ±ź×¼×“æö£©£¬BÖŠŹ£Óą¹ĢĢåµÄÖŹĮæĪŖc g£¬ŌņĀĮµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ_______________”£
£Ø6£©ŹµŃé¹ż³ĢÖŠ£¬ČōĪ“Ļ“µÓ¹żĀĖĖłµĆµÄ²»ČÜĪļ£¬Ōņ²āµĆĀĮµÄÖŹĮæ·ÖŹż½«________£ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°²»ŹÜÓ°Ļģ”±£©”£
(1)NaOHČÜŅŗ
(2)³żČ„ĀĮĆ¾ŗĻ½š±ķĆęµÄŃõ»ÆĤ
(3)¢Ż¢Ł¢Ü¢Ū¢Ś Ź¹DŗĶCµÄŅŗĆęĻąĘ½
(4)2Al+2NaOH+2H2O![]() 2NaAlO2+3H2”ü»ņ2Al+2OH-+2H2O
2NaAlO2+3H2”ü»ņ2Al+2OH-+2H2O![]() 2
2![]() +3H2ӟ
+3H2ӟ
(5)![]()
(6)Ę«Š”
½āĪö£ŗ£Ø1£©æÉøł¾ŻMg”¢AlŠŌÖŹµÄ²»Ķ¬µćŃ”ŌńŹŌ¼Į£¬AlæÉÓėNaOHČÜŅŗ·“Ó¦£¬¶ųMg²»æÉŅŌ£¬¹ŹŃ”ŌńNaOHČÜŅŗ”£
£Ø2£©½«Ć¾ĀĮŗĻ½šŌŚĻ”ĖįÖŠ½žÅŻĘ¬æĢ£¬ÄæµÄŹĒĪŖĮĖ³żČ„ŗĻ½š±ķĆęµÄŃõ»ÆĤ”£
£Ø3£©ŌŚŹµŃéĒ°Ó¦¼ģŃé×°ÖĆĘųĆÜŠŌ£¬²¢¼ĒĀ¼CÖŠŅŗĆęĪ»ÖĆ£¬ŌŁÓÉAĻņB.ÖŠ¼ÓČė×ćĮæNaOHČÜŅŗ£¬Ö±µ½·“Ó¦½įŹų£¬²¢ĒŅ»Öø“µ½ŹŅĪĀ£¬ŌŁ“Ī¼ĒĀ¼CÖŠŅŗĆęĪ»ÖĆ£¬×īŗó½«Ź£Óą¹ĢĢåĻ“µÓ”¢øÉŌļ”¢³ĘÖŲ£¬¹ŹĖ³ŠņĪŖ¢Ż¢Ł¢Ü¢Ū¢Ś£»¶ĮŹżŹ±³żĮĖŹÓĻßĘ½ŹÓĶā£¬»¹ŅŖ×¢ŅāC”¢DĮ½¹ÜÄŚŅŗĆęĻąĘ½£¬²»ŅŖ²śÉśŅŗĆę²ī£¬Ó°Ļģ¶ĮŹż”£
£Ø4£©AlÓėNaOHČÜŅŗ·¢Éś·“Ó¦£ŗ2Al+2NaOH+2H2O![]() 2NaAlO2+3H2”ü
2NaAlO2+3H2ӟ
»ņ2Al+2OH-+2H2O![]() 2
2![]() +3H2ӟ
+3H2ӟ
£Ø5£©m£ØAl£©=£Øa-c£©g£¬n£ØAl£©=![]() n£ØH2£©=
n£ØH2£©=![]() ”Į
”Į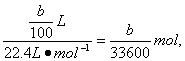
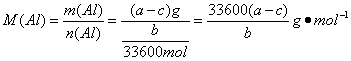 AlµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ
AlµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ![]() ”£
ӣ
£Ø6£©ČōĪ“Ļ“µÓ¹żĀĖĖłµĆ²»ČÜĪļ£¬²āµĆcֵʫ“ó£¬m£ØAl£©=£Øa-c£© gĘ«Š”£¬ŌņAlµÄĻą¶ŌŌ×ÓÖŹĮæֵʫŠ””£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
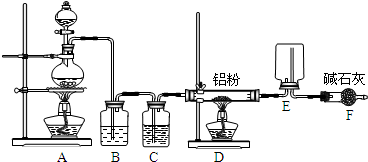
£Ø8·Ö£©Ä³Ń§Ļ°Š”×éÓĆĻĀĶ¼×°ÖĆ²ā¶ØĀĮĆ¾ŗĻ½šÖŠĀĮµÄÖŹĮæ·ÖŹżŗĶĀĮµÄĻą¶ŌŌ×ÓÖŹĮæ.
£ØĢįŹ¾£ŗĆ¾”¢ĀĮ¾łÓėĖį·“Ó¦£¬ĀĮ»¹ÄÜŗĶ¼ī·“Ó¦£ŗ2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£©
ŹµŃéĒ°£¬ĻČ½«ĀĮĆ¾ŗĻ½šŌŚĻ”ĖįÖŠ½žÅŻĘ¬æĢ£¬ĘäÄæµÄŹĒ³żČ„ĀĮĆ¾ŗĻ½š±ķĆęµÄŃõ»ÆĤ”£
£Ø1£©AÖŠŹŌ¼ĮĪŖ .
£ØĢī”°NaOHČÜŅŗ”±»ņ”°Ļ”ŃĪĖį”±£©
£Ø2£©¼ģ²éĘųĆÜŠŌ£¬½«Ņ©Ę·ŗĶĖ®×°Čėø÷ŅĒĘ÷ÖŠ£¬Į¬½ÓŗĆ×°ÖĆŗ󣬊č½ųŠŠµÄ²Ł×÷»¹ÓŠ£ŗ¢Ł¼ĒĀ¼
CµÄŅŗĆęĪ»ÖĆ£»¢Ś½«BÖŠŹ£Óą¹ĢĢå¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ£¬³ĘÖŲ£»¢Ū“żBÖŠ²»ŌŁÓŠĘųĢå²śÉś²¢»Öø“ÖĮŹŅĪĀ£»¢ÜÓÉAĻņBÖŠµĪ¼Ó×ćĮæŹŌ¼Į£»¢Ż¼ģ²éĘųĆÜŠŌ£»¢Žµ÷ÕūĻš½ŗČķ¹ÜŹ¹DŗĶCµÄŅŗĆęĻąĘ½”£
ÉĻŹö²Ł×÷µÄĖ³ŠņŹĒ £»£ØĢīŠņŗÅ£©
£Ø3£©BÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ .
£Ø4£©ŹµŃé¹ż³ĢÖŠ£¬ČōĪ“Ļ“µÓ¹żĀĖĖłµĆµÄ²»ČÜĪļ£¬Ōņ²āµĆĀĮµÄÖŹĮæ·ÖŹż½« .
£ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°²»ŹÜÓ°Ļģ”±£©
£Ø5£©ČōŹµŃéÓĆĀĮĆ¾ŗĻ½šµÄÖŹĮæĪŖa g,²āµĆĒāĘųĢå»żĪŖb ml£ØŅŃ»»ĖćĪŖ±ź×¼×“æö£©£¬BÖŠŹ£Óą¹ĢĢåµÄÖŹĮæĪŖc g£¬ŌņĀĮµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ .£ØÓĆŗ¬a”¢b”¢cµÄ“śŹżŹ½±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÉĀĪ÷Ź”³¤°²Ņ»ÖŠøßČżÉĻѧʌµŚ¶ž“ĪÖŹĮæ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©Ä³Ń§Ļ°Š”×éÓĆĻĀĶ¼×°ÖĆ²ā¶ØĀĮĆ¾ŗĻ½šÖŠĀĮµÄÖŹĮæ·ÖŹżŗĶĀĮµÄĻą¶ŌŌ×ÓÖŹĮ攣
£Ø1£©AÖŠŹŌ¼ĮĪŖ______________”£
£Ø2£©ŹµŃéĒ°£¬ĻČ½«ĀĮĆ¾ŗĻ½šŌŚĻ”ĖįÖŠ½žÅŻĘ¬æĢ£¬ĘäÄæµÄŹĒ_________________________”£
£Ø3£©¼ģ²éĘųĆÜŠŌ£¬½«Ņ©Ę·ŗĶĖ®×°Čėø÷ŅĒĘ÷ÖŠ£¬Į¬½ÓŗĆ×°ÖĆŗ󣬊č½ųŠŠµÄ²Ł×÷»¹ÓŠ£ŗ¢Ł¼ĒĀ¼CµÄŅŗĆęĪ»ÖĆ£»¢Ś½«BÖŠŹ£Óą¹ĢĢå¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬³ĘÖŲ£»¢Ū“żBÖŠ²»ŌŁÓŠĘųĢå²śÉś²¢»Öø“ÖĮŹŅĪĀŗ󣬼ĒĀ¼CµÄŅŗĆęĪ»ÖĆ£»¢ÜÓÉAĻņBÖŠµĪ¼Ó×ćĮæŹŌ¼Į£»¢Ż¼ģ²éĘųĆÜŠŌ”£ÉĻŹö²Ł×÷µÄĖ³ŠņŹĒ___________£»£ØĢīŠņŗÅ£©¼ĒĀ¼CµÄŅŗĆęĪ»ÖĆŹ±£¬³żŹÓĻßĘ½ŹÓĶā£¬»¹Ó¦_______________”£
£Ø4£©BÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________________________”£
£Ø5£©ČōŹµŃéÓĆĀĮĆ¾ŗĻ½šµÄÖŹĮæĪŖa g£¬²āµĆĒāĘųĢå»żĪŖb mL£ØŅŃ»»ĖćĪŖ±ź×¼×“æö£©£¬BÖŠŹ£Óą¹ĢĢåµÄÖŹĮæĪŖc g£¬ŌņĀĮµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ_______________”£
£Ø6£©ŹµŃé¹ż³ĢÖŠ£¬ČōĪ“Ļ“µÓ¹żĀĖĖłµĆµÄ²»ČÜĪļ£¬Ōņ²āµĆĀĮµÄÖŹĮæ·ÖŹż½«________£ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°²»ŹÜÓ°Ļģ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ÄźĮÉÄžŹ”æŖŌøßÖŠøßŅ»ÉĻѧʌµŚŅ»“Īæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø8·Ö£©Ä³Ń§Ļ°Š”×éÓĆĻĀĶ¼×°ÖĆ²ā¶ØĀĮĆ¾ŗĻ½šÖŠĀĮµÄÖŹĮæ·ÖŹżŗĶĀĮµÄĻą¶ŌŌ×ÓÖŹĮæ.
£ØĢįŹ¾£ŗĆ¾”¢ĀĮ¾łÓėĖį·“Ó¦£¬ĀĮ»¹ÄÜŗĶ¼ī·“Ó¦£ŗ2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£©
ŹµŃéĒ°£¬ĻČ½«ĀĮĆ¾ŗĻ½šŌŚĻ”ĖįÖŠ½žÅŻĘ¬æĢ£¬ĘäÄæµÄŹĒ³żČ„ĀĮĆ¾ŗĻ½š±ķĆęµÄŃõ»ÆĤ”£
£Ø1£©AÖŠŹŌ¼ĮĪŖ .
£ØĢī”°NaOHČÜŅŗ”±»ņ”°Ļ”ŃĪĖį”±£©
£Ø2£©¼ģ²éĘųĆÜŠŌ£¬½«Ņ©Ę·ŗĶĖ®×°Čėø÷ŅĒĘ÷ÖŠ£¬Į¬½ÓŗĆ×°ÖĆŗ󣬊č½ųŠŠµÄ²Ł×÷»¹ÓŠ£ŗ¢Ł¼ĒĀ¼
CµÄŅŗĆęĪ»ÖĆ£»¢Ś½«BÖŠŹ£Óą¹ĢĢå¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ£¬³ĘÖŲ£»¢Ū“żBÖŠ²»ŌŁÓŠĘųĢå²śÉś²¢»Öø“ÖĮŹŅĪĀ£»¢ÜÓÉAĻņBÖŠµĪ¼Ó×ćĮæŹŌ¼Į£»¢Ż¼ģ²éĘųĆÜŠŌ£»¢Žµ÷ÕūĻš½ŗČķ¹ÜŹ¹DŗĶCµÄŅŗĆęĻąĘ½”£
ÉĻŹö²Ł×÷µÄĖ³ŠņŹĒ £»£ØĢīŠņŗÅ£©
£Ø3£©BÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ .
£Ø4£©ŹµŃé¹ż³ĢÖŠ£¬ČōĪ“Ļ“µÓ¹żĀĖĖłµĆµÄ²»ČÜĪļ£¬Ōņ²āµĆĀĮµÄÖŹĮæ·ÖŹż½« .
£ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°²»ŹÜÓ°Ļģ”±£©
£Ø5£©ČōŹµŃéÓĆĀĮĆ¾ŗĻ½šµÄÖŹĮæĪŖa g,²āµĆĒāĘųĢå»żĪŖb ml£ØŅŃ»»ĖćĪŖ±ź×¼×“æö£©£¬BÖŠŹ£Óą¹ĢĢåµÄÖŹĮæĪŖc g£¬ŌņĀĮµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ .£ØÓĆŗ¬a”¢b”¢cµÄ“śŹżŹ½±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com