
֊ѧ³£¼ūµÄij·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖa£«bØD”śc£«d£«H2O(Ī“ÅäĘ½£¬·“Ó¦Ģõ¼žŅŃĀŌČ„)”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ČōaŹĒĢś£¬bŹĒĻ”ĻõĖį(¹żĮæ)£¬ĒŅaæÉČÜÓŚcČÜŅŗÖŠ”£ŌņaÓėb·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
___________________________ӣ
(2)Čōc”¢dĪŖĘųĢ壬ĒŅ¶¼ÄÜŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬Ōņ½«“Ė»ģŗĻĘųĢåĶØČėäåĖ®ÖŠ£¬³ČÉ«ĶŹČ„£¬Š“³öĘäĶŹÉ«¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ___________________________”£
(3)ČōcŹĒĪŽÉ«ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬ĘäĖ®ČÜŅŗĻŌČõ¼īŠŌ£¬ŌŚ±ź×¼×“æöĻĀÓĆÅÅæÕĘų·ØŹÕ¼ÆcĘųĢ壬µĆĘ½¾łÄ¦¶ūÖŹĮæĪŖ20 g”¤mol£1µÄ»ģŗĻĘųĢå½ųŠŠÅēČŖŹµŃ锣¼ŁÉčČÜÖŹ²»Ą©É¢£¬ŹµŃéĶź³ÉŗóÉÕĘæÖŠĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________mol”¤L£1(Š”Źżµćŗó±£Įō2Ī»ÓŠŠ§Źż×Ö)”£
(4)ČōaŹĒŌģ³ÉĪĀŹŅŠ§Ó¦µÄÖ÷ŅŖĘųĢ壬c”¢d¾łĪŖÄĘŃĪ£¬²Ī¼Ó·“Ó¦µÄa”¢bµÄĪļÖŹµÄĮæÖ®±ČĪŖ4:5”£ŌņÉĻŹö·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀ±ķÖŠ£¬¶Ō³ĀŹöI”¢¢ņµÄÕżČ·ŠŌ¼°Į½Õß¼äŹĒ·ń¾ßÓŠŅņ¹ū¹ŲĻµµÄÅŠ¶Ļ¶¼ÕżČ·µÄŹĒ
| Ń”Ļī | ³ĀŹöI | ³ĀŹö¢ņ | ÅŠ¶Ļ |
| A | KSCNČÜŅŗæɼģŃéFe3+ | “ż²āŅŗĻČŗóµĪČė×ćĮæĀČĖ®ŗĶ¼øµĪKSCNČÜŅŗŗó±äĪŖŃŖŗģÉ«£¬Ö¤Ć÷“ż²āŅŗŗ¬Fe3+ | I¶Ō£»¢ņ¶Ō£»ĪŽ |
| B | SO2¾ßÓŠ»¹ŌŠŌ | SO2ĶØČėBa(NO3)2ČÜŅŗÖŠæɼū°×É«³ĮµķÉś³É | I¶Ō£»¢ņ¶Ō£»ÓŠ |
| C | NO2ŗĶäåÕōĘų¶¼³Źŗģ×ŲÉ« | ÓĆĖ®æɼų±šNO2ŗĶäåÕōĘų | I¶Ō£»¢ņ“ķ£»ĪŽ |
| D | ·“Ó¦Īļ±ČĄż²»Ķ¬æɵ¼ÖĀ²śĪļ²»Ķ¬ | NaÓėO2·“Ó¦æÉÄÜÉś³ÉNa2O£¬Ņ²æÉÄÜÉś³ÉNa2O2 | I¶Ō£»¢ņ¶Ō£»ÓŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FĮłÖÖ¶ĢÖÜĘŚŌŖĖŲÖŠ£¬A”¢B”¢C”¢DŹĒ×é³Éµ°°×ÖŹµÄ»ł±¾ŌŖĖŲ£»AÓėBµÄŌ×ÓŠņŹżÖ®ŗĶµČÓŚCŌ×ÓŗĖÄŚµÄÖŹ×ÓŹż£»AÓėE”¢DÓėF·Ö±šĪ»ÓŚĶ¬Ņ»Ö÷×壬ĒŅFŌ×ÓŗĖÄŚµÄÖŹ×ÓŹżŹĒDŌ×ÓŗĖĶāµē×ÓŹżµÄ2±¶”£¾Ż“Ė£¬Ēė»Ų“š£ŗ
£Ø1£©FŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ ”£
£Ø2£©ÓÉA”¢C”¢D”¢F°“8:2:4:1Ō×ÓøöŹż±Č×é³ÉµÄ»ÆŗĻĪļ¼×ÖŠŗ¬ÓŠµÄ»Æѧ¼üĄąŠĶĪŖ £»¼×ČÜŅŗÖŠø÷Ąė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ £ØÓĆĄė×ÓÅØ¶Č·ūŗűķŹ¾£©”£
£Ø3£©»ÆŗĻĪļŅŅÓÉA”¢C×é³ÉĒŅĻą¶Ō·Ö×ÓÖŹĮæĪŖ32£»»ÆŗĻĪļ±ūÓÉA”¢D×é³ÉĒŅ·Ö×ÓÄŚµē×Ó×ÜŹżÓėŅŅ·Ö×ÓÄŚµē×Ó×ÜŹżĻąµČ£»ŅŅÓė±ūµÄ·“Ó¦æÉÓĆÓŚ»š¼ż·¢Éä£Ø·“Ó¦²śĪļ²»ĪŪČ¾“óĘų£©£¬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ÓÉA”¢D”¢E”¢F×é³ÉµÄ»ÆŗĻĪļ¶”ÄÜÓėĮņĖį·“Ó¦²¢·Å³ö“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬Ōņ¶”µÄ»ÆѧŹ½ĪŖ £»ŹµŃé²āµĆ¶”ČÜŅŗĻŌČõĖįŠŌ£¬ÓÉ“ĖÄćÄÜµĆ³öµÄ½įĀŪŹĒ ”£
£Ø5£©ÓÉB”¢A°“1:4Ō×ÓøöŹż±Č×é³ÉµÄ»ÆŗĻĪļĪģÓėDµÄ³£¼ūĘųĢ¬µ„ÖŹ¼°NaOHČÜŅŗ¹¹³ÉŌµē³Ų£ØČēĶ¼£©£¬ŹŌ·ÖĪö£ŗ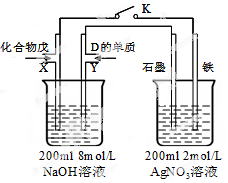
¢Ł±ÕŗĻK£¬Š“³ö×ó³ŲXµē¼«µÄ·“Ó¦Ź½ £»
¢Ś±ÕŗĻK£¬µ±Xµē¼«ĻūŗÄ1.6g»ÆŗĻĪļĪģŹ±£Ø¼ŁÉč¹ż³ĢÖŠĪŽČĪŗĪĖšŹ§£©£¬ŌņÓŅ³ŲĮ½¼«¹²·Å³öĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ Éż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢XŹĒ֊ѧ»Æѧ³£¼ūĪļÖŹ,¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É,×Ŗ»Æ¹ŲĻµČēĶ¼”£ĒėÕė¶ŌŅŌĻĀČżÖÖ²»Ķ¬Ēéæö»Ų“š: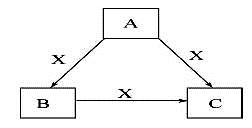
£Ø1£©ČōA”¢B”¢CÖŠ¾łŗ¬Ķ¬Ņ»ÖÖ³£¼ū½šŹōŌŖĖŲ,øĆŌŖĖŲŌŚCÖŠŅŌŅõĄė×ÓŠĪŹ½“ęŌŚ,½«A”¢CµÄĖ®ČÜŅŗ»ģŗĻæɵĆBµÄ°×É«½ŗד³Įµķ”£
¢ŁAÖŠŗ¬ÓŠµÄ½šŹōŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ__________£¬ĻņĖ®ÖŠ¼ÓČėXĪļÖŹ£¬X¶ŌĖ®µÄµēĄėĘ½ŗāµÄÓ°ĻģŹĒ £ØĢī”°“Ł½ų”±”¢”°ŅÖÖĘ”±»ņ”°ĪŽÓ°Ļģ”±£© ”£
¢ŚAÓėCµÄĖ®ČÜŅŗ»ģŗĻŗóÉś³ÉB·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©ČōAĪŖ¹ĢĢ¬·Ē½šŹōµ„ÖŹ,AÓėXĶ¬ÖÜĘŚ,Ķ¬Ź±AŌŚXÖŠČ¼ÉÕ,²śÉś°×É«ŃĢĪķ,³£ĪĀ³£Ń¹ĻĀCĪŖ°×É«¹ĢĢå,B·Ö×ÓÖŠø÷Ō×Ó×īĶā²ć¾łĪŖ8µē×Ó½į¹¹”£
¢ŁČōAĪŖøĆŌŖĖŲµÄ°×É«¹ĢĢåµ„ÖŹ£¬Ōņ1mol Aµ„ÖŹÖŠŗ¬¹²¼Ū¼üŹżÄæĪŖ NA ,BµÄµē×ÓŹ½ĪŖ___________”£
¢ŚXÓėĖ®·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©ČōA”¢B”¢CµÄŃęÉ«·“Ó¦³Ź»ĘÉ«,Ė®ČÜŅŗ¾łĪŖ¼īŠŌ,³£ĪĀĻĀ,XĪŖĘųĢ¬ĖįŠŌŃõ»ÆĪļ”£
¢ŁAÖŠĖłŗ¬ÓŠµÄ»Æѧ¼üĄąŠĶŹĒ_____________________”£
¢ŚCČÜŅŗÖŠĄė×ÓÅضČÓÉŠ”µ½“óµÄĖ³ŠņŹĒ__ _________________ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾£ŗ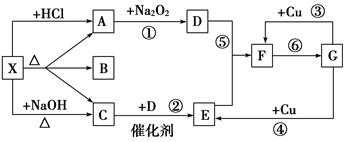
Ķ¼ÖŠĆæŅ»·½øń±ķŹ¾ÓŠ¹ŲµÄŅ»ÖÖ·“Ó¦Īļ»ņÉś³ÉĪļ£¬Éś³ÉĪļA”¢B”¢CµÄĪļÖŹµÄĮæĻąµČ£¬ĘäÖŠA”¢CĪŖĪŽÉ«ĘųĢ唣ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©ĪļÖŹXŹĒ________£¬CŹĒ________£¬FŹĒ________£¬GŹĒ ”£
£Ø2£©·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ
_______________________________________________________________________ӣ
£Ø3£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ
________________________________________________________________________ӣ
£Ø4£©·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½ŹĒ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹¤ŅµÉĻÓÉ»ĘĶæó(Ö÷ŅŖ³É·ÖCuFeS2)Ņ±Į¶ĶµÄÖ÷ŅŖĮ÷³ĢČēĻĀ£ŗ
£Ø1£©ĘųĢåAÖŠµÄ“óĘųĪŪČ¾ĪļæÉŃ”ÓĆĻĀĮŠŹŌ¼ĮÖŠµÄ_______ĪüŹÕ”£
a£®ÅØH2SO4 b£®Ļ”HNO3 c£®NaOHČÜŅŗ d£®°±Ė®
£Ø2£©ÓĆĻ”H2SO4½žÅŻČŪŌüB£¬Č”ÉŁĮæĖłµĆČÜŅŗ£¬µĪ¼ÓKSCNČÜŅŗŗó³ŹŗģÉ«£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚ (ĢīĄė×Ó·ūŗÅ)£¬¼ģŃéČÜŅŗÖŠ»¹“ęŌŚFe2£«µÄ·½·ØŹĒ (×¢Ć÷ŹŌ¼Į”¢ĻÖĻó)”£
£Ø3£©ÓÉÅŻĶŅ±Į¶“ÖĶµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø4£©ŅŌCuSO4ČÜŅŗĪŖµē½āÖŹČÜŅŗ½ųŠŠ“ÖĶ(ŗ¬Al”¢Zn”¢Ag”¢Pt”¢AuµČŌÓÖŹ)µÄµē½ā¾«Į¶£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£
a£®µēÄÜČ«²æ×Ŗ»ÆĪŖ»ÆѧÄÜ
b£®“ÖĶ½ÓµēŌ“Õż¼«£¬·¢ÉśŃõ»Æ·“Ó¦
c£®ČÜŅŗÖŠCu2£«ĻņŃō¼«ŅʶÆ
d£®ĄūÓĆŃō¼«ÄąæÉ»ŲŹÕAg”¢Pt”¢AuµČ½šŹō
£Ø5£©ĄūÓĆ·“Ó¦2Cu+O2+2H2SO4=2CuSO4+2H2OæÉÖʱøCuSO4£¬Čō½«øĆ·“Ó¦Éč¼ĘĪŖŌµē³Ų£¬ĘäÕż¼«µē¼«·“Ó¦Ź½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓūĢ½¾æijæóŹÆæÉÄÜŹĒÓÉFeCO3”¢SiO2”¢Al2O3ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ×é³É£¬Ģ½¾æ¹ż³ĢČēĻĀĶ¼ĖłŹ¾”£ŅŃÖŖ£ŗĢ¼Ėį²»ÄÜČܽāAl(OH)3³Įµķ”£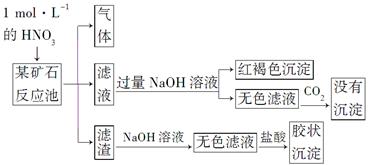
(1)SiŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ___________________________________”£
(2)ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£
a£®ĖįŠŌ£ŗH2CO3>H2SiO3
b£®Ō×Ó°ė¾¶£ŗO<C<Si<Al
c£®ĪČ¶ØŠŌ£ŗH2O>CH4>SiH4
d£®Ąė×Ó°ė¾¶£ŗO2£<Al3£«
(3)øĆæóŹÆµÄ×é³ÉŹĒ________£¬ĀĖŌüŗĶNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________________________________________________”£
(4)øĆæóŹÆŗĶ1 mol”¤L£1HNO3·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
___________________________________________________________ӣ
(5)¹¤ŅµÉĻŅĄ¾ŻÉĻŹöŹµŃéŌĄķ“¦ĄķøĆæóŹÆ£¬½«·“Ó¦³ŲŅŻ³öµÄĘųĢåÓėŅ»¶ØĮæµÄO2»ģŗĻŃ»·ĶØČė·“Ó¦³ŲÖŠ”£ÄæµÄŹĒ____________________________________£»
Čō“¦ĄķøĆæóŹÆ2.36”Į103 kg£¬µĆµ½ĀĖŌü1.2”Į103 kg£¬ĄķĀŪÉĻÖĮÉŁŠčŅŖ1 mol”¤L£1 HNO3µÄĢå»żĪŖ________L”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øł¾ŻŅŖĒóĶź³ÉĻĀĮŠø÷Š”Ģā
£Ø1£©¢ŁŹµŃéŹŅÓĆ¼ÓČČ¹ĢĢå»ģŗĻĪļµÄ·½·ØÖʱø°±ĘųµÄ»Æѧ·“Ó¦·½³ĢŹ½ŹĒ ”£
¢ŚĪŖĮĖµĆµ½øÉŌļµÄNH3£¬ÓĆ________×öøÉŌļ¼Į”££ØĢī±ąŗÅ£©
| A£®¼īŹÆ»Ņ | B£®ÅØH2SO4 | C£®ĪŽĖ®CaCl2 | D£®P2O5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĪļÖŹX”¢Y”¢Z¾łŗ¬Ķ¬ÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬Ęä×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£Ø·“Ó¦Ģõ¼žĪ“±ź³ö£©”£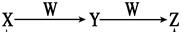
£Ø1£©ČōXŹĒĪŽÉ«¼«Ņ×ČÜÓŚĖ®µÄ“Ģ¼¤ŠŌĘųĪ¶ĘųĢ壬ZŹĒŗģ×ŲÉ«ĘųĢ壬ÓÉYÓėW·“Ӧɜ³ÉZµÄ»Æѧ·½³ĢŹ½ŹĒ_______________________________________________________”£
£Ø2£©ČōXŗ¬ČżÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĘäÖŠĮ½ÖÖŌŖĖŲµÄŌ×ÓµÄÖŹ×ÓŹżÖ®ŗĶµČÓŚĮķŅ»ÖÖŌŖĖŲŌ×ÓµÄÖŹ×ÓŹż£¬µ„ÖŹWŹĒ³£¼ū½šŹō£¬ŌņXµÄĻ”ČÜŅŗ×Ŗ»ÆĪŖYµÄĄė×Ó·½³ĢŹ½ŹĒ
ӣ
£Ø3£©ČōXŹĒæÕĘųµÄÖ÷ŅŖ³É·ÖÖ®Ņ»£¬WŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒÄŚ²ćµē×ÓŹżµÄ¶ž±¶£»ŌņYÓėW·“Ӧɜ³É0.1 mol ZŹ±£¬·“Ó¦ÖŠ×ŖŅʵĵē×ÓŹżĪŖ__________________________”£
£Ø4£©ČōYŹĒ°×É«½ŗד³Įµķ£¬WĪŖÉÕ¼ī£¬Ōņ0.1mol YÓė×ćĮæW·“Ӧɜ³ÉZŹ±ĻūŗÄÉÕ¼īµÄÖŹĮæĪŖ æĖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com