
”¾ĢāÄæ”æŌŚŗćĪĀ”¢ŗćČŻĻĀ£¬ÓŠ·“Ó¦2A(g)£«2B(g)![]() C(g)£«3D(g)£¬ĻÖ“ÓĮ½ĢõĶ¾¾¶·Ö±š½ØĮ¢Ę½ŗā”£Ķ¾¾¶¢ń£ŗA”¢BµÄĘšŹ¼ÅØ¶Č¾łĪŖ2mol”¤L£1£»Ķ¾¾¶¢ņ£ŗC”¢DµÄĘšŹ¼ÅØ¶Č·Ö±šĪŖ2mol”¤L£1ŗĶ6mol”¤L£1”£ŅŌĻĀŠšŹöÕżČ·µÄŹĒ(””””)
C(g)£«3D(g)£¬ĻÖ“ÓĮ½ĢõĶ¾¾¶·Ö±š½ØĮ¢Ę½ŗā”£Ķ¾¾¶¢ń£ŗA”¢BµÄĘšŹ¼ÅØ¶Č¾łĪŖ2mol”¤L£1£»Ķ¾¾¶¢ņ£ŗC”¢DµÄĘšŹ¼ÅØ¶Č·Ö±šĪŖ2mol”¤L£1ŗĶ6mol”¤L£1”£ŅŌĻĀŠšŹöÕżČ·µÄŹĒ(””””)
A. “ļµ½Ę½ŗāŹ±£¬Ķ¾¾¶¢ńµÄ·“Ó¦ĖŁĀŹµČÓŚĶ¾¾¶¢ņµÄ·“Ó¦ĖŁĀŹ
B. “ļµ½Ę½ŗāŹ±£¬Ķ¾¾¶¢ńĖłµĆ»ģŗĻĘųĢåµÄŃ¹ĒæµČÓŚĶ¾¾¶¢ņĖłµĆ»ģŗĻĘųĢåµÄŃ¹Ēæ
C. Į½Ķ¾¾¶×īÖÕ“ļµ½Ę½ŗāŹ±£¬ĢåĻµÄŚø÷×é·ÖµÄ°Ł·Öŗ¬ĮæĻąĶ¬
D. Į½Ķ¾¾¶×īÖÕ“ļµ½Ę½ŗāŹ±£¬ĢåĻµÄŚø÷×é·ÖµÄ°Ł·Öŗ¬Įæ²»ĻąĶ¬
”¾“š°ø”æC
”¾½āĪö”æ·“Ó¦2A£Øg£©+2B£Øg£©C£Øg£©+3D£Øg£©æÉÖŖ£¬·“Ó¦Ē°ŗóĘųĢåµÄ»Æѧ¼ĘĮæŹżĻąµČ£¬Ń¹Ēæ¶ŌĘ½ŗāŅʶÆƻӊӰĻģ£¬µ±Āś×ć¢ņĖł¼ÓĪļÖŹĶźČ«×Ŗ»ÆĪŖA”¢BŹ±£¬Óė¢ńĪļÖŹµÄĮæ±ČÖµĻąµČ£¬A”¢¢ņĶ¾¾¶“ļµ½Ę½ŗāŹ±ÅØ¶Č“ó£¬Ń¹Ēæ“󣬷“Ó¦ĖŁĀŹÓ¦½Ļ“󣬹ŹA“ķĪó£»B”¢¢ņĶ¾¾¶“ļµ½Ę½ŗāŹ±ÅØ¶Č“ó£¬Ń¹Ēæ“󣬷“Ó¦ĖŁĀŹÓ¦½Ļ“󣬹ŹB“ķĪó£»C”¢¢ń”¢¢ņĮ½Ķ¾¾¶×īÖÕ“ļµ½ĻąĶ¬Ę½ŗāדĢ¬£¬ĢåĻµÄŚ»ģŗĻĘųµÄ°Ł·Ö×é³ÉĻąĶ¬£¬¹ŹCÕżČ·£»D”¢Į½ÖÖĶ¾¾¶Ę½ŗāדĢ¬ĻąĶ¬£¬ø÷ĪļÖŹµÄŗ¬ĮæĻąĶ¬£¬ŌņĢåĻµÄŚ»ģŗĻĘųµÄ°Ł·Ö×é³ÉĻąĶ¬£¬¹ŹD“ķĪó£»¹ŹŃ”C”£


 °ŁÄźŃ§µäæĪŹ±Ń§Į·²āĻµĮŠ“š°ø
°ŁÄźŃ§µäæĪŹ±Ń§Į·²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅÓĆNaOH¹ĢĢåÅäÖĘ245 mL 1.2 mol/LµÄNaOHČÜŅŗ£¬ĢīæÕ²¢Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÅäÖĘ245 mL 1.2 mol/LµÄNaOHČÜŅŗ£¬ŠčÓĆĶŠÅĢĢģĘ½³ĘČ”NaOH______g£¬ĖłŠčµÄ²£Į§ŅĒĘ÷ÓŠĮæĶ²”¢ÉÕ±”¢______________________________________”£
£Ø2£©ČŻĮæĘæÉĻŠč±źÓŠŅŌĻĀĪåĻīÖŠµÄ_____________________”££ØĢīŠņŗÅ£©
¢ŁĪĀ¶Č ¢ŚÅØ¶Č ¢ŪČŻĮæ ¢ÜŃ¹Ēæ ¢ŻæĢ¶ČĻß
£Ø3£©ŌŚ¶ØČŻ²Ł×÷Ź±£¬Ó¦½«ÕōĮóĖ®×¢ČėČŻĮæĘ棬ŅŗĆęĄėČŻĮæĘæ¾±æĢ¶ČĻß______________“¦£¬øÄÓĆ____________µĪ¼ÓÕōĮóĖ®ÖĮÓėæĢ¶ČĻßĻąĘ½”£øĒŗĆĘæČū£¬·“ø“µßµ¹Ņ”ŌČ”£
£Ø4£©±¾ŹµŃéÖŠ£¬ĻĀĮŠÅäÖʵÄČÜŅŗÅضČĘ«µĶµÄŹĒ_____________”£ £ØĢī×ÖÄø“śŗÅ£©
A£®³ĘĮæNaOHŹ±£¬Ź¹ÓĆÓĪĀė£¬ķĄĀė“ķ·ÅŌŚ×óÅĢ
B£®ĻņČŻĮæĘæÖŠ×ŖŅĘČÜŅŗŹ±£¬²»É÷ÓŠŅŗµĪČ÷ŌŚČŻĮæĘæĶāĆę
C£®¶ØČŻŹ±ŃöŹÓ¹Ū²ģŅŗĆę
D£®ČܽāŗóĪ“ĄäČ“µ½ŹŅĪĀ¾Ķ½«ČÜŅŗ×ŖŅʵ½ČŻĮæĘæÖŠ
E£®ÅäÖĘĒ°£¬ČŻĮæĘæÖŠÓŠÉŁĮæÕōĮóĖ®
£Ø5£©ĻĀĮŠ¹ŲÓŚČŻĮæĘæµÄŹ¹ÓĆ·½·ØŗĶ²Ł×÷µÄĆčŹö£¬ÕżČ·µÄŹĒ___________”££ØĢī×ÖÄø“śŗÅ£©
A£®Ź¹ÓĆČŻĮæĘæĒ°¼ģ²éĖüŹĒ·ńĀ©Ė®
B£®ŅżĮ÷Ź±£¬²£Į§°ōµÄĻĀ¶ĖÓ¦ŌŚČŻĮæĘææĢ¶ČĻßŅŌÉĻ
C£®ÅäÖĘČÜŅŗŹ±£¬Čē¹ūŹŌŃłŹĒ¹ĢĢ壬°Ń³ĘŗƵďŌŃłÓĆÖ½ĢõŠ”ŠÄµ¹ČėČŻĮæĘæÖŠ£¬¶ØČŻÖĮæĢ¶ČĻß
D£®ÅäÖĘČÜŅŗŹ±£¬ČōŹŌŃłŹĒŅŗĢ壬ÓĆĮæĶ²Č”ŹŌŃłŗóÖ±½Óµ¹ČėČŻĮæĘæÖŠ£¬»ŗĀż¼ÓĖ®¶ØČŻÖĮæĢ¶ČĻß
E£®øĒŗĆĘæČū£¬ÓĆŹ³Öø¶„×”ĘæČū£¬ĮķŅ»Ö»ŹÖĶŠ×”Ęæµ×£¬°ŃČŻĮæĘæ·“ø“µ¹×Ŗ¶ą“Ī£¬Ņ”ŌČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĒāĘųŹĒŅ»ÖÖĄķĻėµÄĀĢÉ«ÄÜŌ“”£
£Ø1£©ŌŚ101KPĻĀ£¬1gĒāĘųĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®·Å³ö142.9kJµÄČČĮ棬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁĒāĘųµÄČ¼ÉÕČČĪŖ______________£»
¢ŚøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ________________________________£»
£Ø2£©ĒāÄÜµÄ“ę“¢ŹĒĒāÄÜĄūÓƵÄĒ°Ģį£¬æĘѧ¼ŅŃŠ¾æ³öŅ»ÖÖ“¢ĒāŗĻ½šMg2Ni£¬ŅŃÖŖ£ŗ
Mg(s)£«H2(g)===MgH2(s) ¦¤H1£½£74.5kJ”¤mol£1£»
Mg2Ni(s)£«2H2(g)===Mg2NiH4(s) ¦¤H2£»
Mg2Ni(s)£«2MgH2(s)===2Mg(s)£«Mg2NiH4(s) ¦¤H3£½+84.6kJ”¤mol£1”£
Ōņ¦¤H2£½____________kJ”¤mol£1£»
ijŹµŃ銔×éÓĆ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/LĮņĖįČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£
£Ø3£©µ¹ČėNaOHČÜŅŗµÄÕżČ·²Ł×÷ŹĒ_______________(“ÓĻĀĮŠŃ”³ö)”£
A£®ŃŲ²£Į§°ō»ŗĀżµ¹Čė B£®Ņ»“ĪŃøĖŁµ¹Čė C£®·ÖČż“ĪÉŁĮæµ¹Čė
(4)Ź¹ĮņĖįÓėNaOHČÜŅŗ»ģŗĻ¾łŌȵÄÕżČ·²Ł×÷ŹĒ_________(“ÓĻĀĮŠŃ”³ö)”£
A£®ÓĆĪĀ¶Č¼ĘŠ”ŠÄ½Į°č B£®½ŅæŖÓ²Ö½Ę¬ÓĆ²£Į§°ō½Į°č
C£®ĒįĒįµŲÕńµ“ÉÕ± D£®ÓĆĢ×ŌŚĪĀ¶Č¼ĘÉĻµÄ»·ŠĪ²£Į§°ō½Į°č°ōĒįĒįµŲ½Į¶Æ
£Ø5£©Č”50 mL NaOHČÜŅŗŗĶ30 mLĮņĖįČÜŅŗ½ųŠŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ”£
¢ŁĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ
ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č t2/”ę | ĪĀ¶Č²īĘ½¾łÖµ £Øt2-t1£©/”ę | |||
H2SO4 | NaOH | Ę½¾łÖµ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
¢Ś½üĖĘČĻĪŖ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/LĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻc=4.18 J/(g”¤”ę£©”£ŌņÖŠŗĶČČ”÷H=______________(Č”Š”ŹżµćŗóŅ»Ī»£©”£
¢ŪÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ŗ___________”£(ČĪŅāŠ“³öŅ»µć£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪļÖŹÖŠ£¬²»ÄÜŹ¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗŗĶøßĆĢĖį¼ŲĖįŠŌČÜŅŗĶŹÉ«µÄŹĒ£Ø £©
A.C2H4B.±ūĻ©C.C5H12D.ŅŅČ²
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ·ØŅ½³£ÓĆĀķŹĻŹŌÉé·Ø¼ģŃéŹĒ·ńÅųĖŖ( As2O3)ÖŠ¶¾£¬Éę¼°µÄ·“Ó¦ČēĻĀ£ŗ
I£ŗ6Zn+As2O3+12HCl=6ZnCl2+2AsH3£ØÉéĶ飩”ü+3H2O
¢ņ£ŗ2AsH3=2As£ØŗŚÉ«Éé¾µ£©+3H2
£Ø1£©Š“³öÉéµÄ»łĢ¬Ō×Ó¼Ūµē×ÓÅŲ¼Ķ¼______________”£
£Ø2£©ÉéĶéµÄæÕ¼ä½į¹¹ĪŖ_______£»ÉéĶéÖŠŠÄŌ×ÓŌӻƷ½Ź½ĪŖ________”£
£Ø3£©ÉéĶéĶ¬×åĶ¬ĻµĮŠĪļÖŹĻą¹ŲŠŌÖŹČēĻĀ±ķ£ŗ
NH3 | PH3 | AsH3 | SbH3 | |
ČŪµć/”ę | -77.8 | -133.5 | -116.3 | -88 |
·Šµć/”ę | -34.5 | -87.5 | -62.4 | -18.4 |
“ÓPH3”śAsH3”śSbH3ČŪ·ŠµćŅĄ“ĪÉżøßµÄŌŅņŹĒ_________£»NH3·Ö×ÓĄżĶāµÄŌŅņŹĒ_______”£
£Ø4£©µŚŅ»µēĄėÄÜŹż¾ŻI(As)>I(Se)£¬æÉÄܵÄŌŅņŹĒ_____________”£
£Ø5£©ÉéÓėī÷(In)ŠĪ³ÉµÄ»ÆŗĻĪļ(X)¾ßÓŠÓÅĮ¼µÄ¹āµēŠŌÄÜ£¬¹ć·ŗÓ¦ÓĆÓŚ¹āĻĖĶØŠÅÓĆ¼¤¹āĘ÷£¬Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾£¬ŌņĘä»ÆѧŹ½ĪŖ______£»¾§°ū±ß³¤a= 666.67pm£¬ŌņĘäĆܶČĪŖ_____g/cm3£Ø±ß³¤aæÉÓĆ![]() ½üĖĘ¼ĘĖć£¬ÉčNA=6.0”Į1023/mol£©”£
½üĖĘ¼ĘĖć£¬ÉčNA=6.0”Į1023/mol£©”£
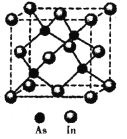
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌĖÓĆ»Æѧ·“Ó¦ŌĄķ·ÖĪö½ā“šŅŌĻĀĪŹĢā”£
(1)ŅŃÖŖ£ŗ
¢ŁCO(g)+2H2(g)![]() CH3OH(g) ”÷H1=-91 kJ”¤mol-1
CH3OH(g) ”÷H1=-91 kJ”¤mol-1
¢Ś2CH3OH(g) ![]() CH3OCH3(g)+H2O(g) ”÷H2=-24 kJ”¤mol-1
CH3OCH3(g)+H2O(g) ”÷H2=-24 kJ”¤mol-1
¢ŪCO(g)+H2O(g)![]() CO2(g)+H2(g) ”÷H3=-41 kJ”¤mol-1
CO2(g)+H2(g) ”÷H3=-41 kJ”¤mol-1
ĒŅČżøö·“Ó¦µÄĘ½ŗā³£ŹżŅĄ“ĪĪŖK1”¢K2”¢K3
Ōņ·“Ó¦3CO(g)+3H2(g)![]() CH3OCH3(g)+CO2(g) ”÷H=_____________£¬»ÆŃ§Ę½ŗā³£ŹżK=_________£ØÓĆŗ¬K1”¢K2”¢K3µÄ“śŹżŹ½±ķŹ¾£©”£
CH3OCH3(g)+CO2(g) ”÷H=_____________£¬»ÆŃ§Ę½ŗā³£ŹżK=_________£ØÓĆŗ¬K1”¢K2”¢K3µÄ“śŹżŹ½±ķŹ¾£©”£
(2)Ņ»¶ØĢõ¼žĻĀ£¬Čō½«Ģå»ż±ČĪŖ1:2µÄCOŗĶH2ĘųĢåĶØČėĢå»żŅ»¶ØµÄĆܱÕČŻĘ÷ÖŠ·¢Éś·“Ó¦£ŗ3CO(g)+3H2(g)![]() CH3OCH3(g)+CO2(g) £¬ĻĀĮŠÄÜĖµĆ÷·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄŹĒ_________”£
CH3OCH3(g)+CO2(g) £¬ĻĀĮŠÄÜĖµĆ÷·“Ó¦“ļµ½Ę½ŗāדĢ¬µÄŹĒ_________”£
a.ĢåĻµŃ¹Ēæ±£³Ö²»±ä b.»ģŗĻĘųĢåĆܶȱ£³Ö²»±ä
c.COŗĶH2µÄĪļÖŹµÄĮæ±£³Ö²»±ä d.COµÄĻūŗÄĖŁĀŹµČÓŚCO2µÄÉś³ÉĖŁĀŹ
(3)°±ĘųČÜÓŚĖ®µĆµ½°±Ė®”£ŌŚ25”ćCĻĀ£¬½«x mol”¤L-1µÄ°±Ė®Óėy mol”¤L-1µÄŃĪĖįµČĢå»ż»ģŗĻ£¬·“Ó¦ŗóČÜŅŗÖŠĻŌÖŠŠŌ£¬Ōņc£ØNH4+£©______c£ØCl-£©£ØĢī”°£¾”±”¢”°£¼”±»ņ”°=”±£©£»ÓĆŗ¬xŗĶyµÄ“śŹżŹ½±ķŹ¾³ö°±Ė®µÄµēĄėĘ½ŗā³£Źż______”£
(4)æĘѧ¼Ņ·¢Ć÷ĮĖŹ¹NH3Ö±½ÓÓĆÓŚČ¼ĮĻµē³ŲµÄ·½·Ø£¬Ęä×°ÖĆÓĆ²¬ŗŚ×÷µē¼«”¢¼ÓČėµē½āÖŹČÜŅŗÖŠ£¬Ņ»øöµē¼«ĶØČėæÕĘų£¬ĮķŅ»µē¼«ĶØČėNH3”£Ęäµē³Ų·“Ó¦Ź½ĪŖ£ŗ4NH3+3O2ØT2N2+6H2O£¬µē½āÖŹČÜŅŗÓ¦ĻŌ______£ØĢī”°ĖįŠŌ”±”¢”°ÖŠŠŌ”±»ņ”°¼īŠŌ”±£©£¬Š“³öÕż¼«µÄµē¼«·“Ó¦·½³ĢŹ½______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĒ°ĖÄÖÜĘŚµÄA”¢B”¢C”¢DĖÄÖÖŌŖĖŲŌŚÖÜĘŚ±ķÖŠ¾łÓėŌŖĖŲX½ōĆÜĻąĮŚ”£ŅŃÖŖŌŖĖŲX×īøß¼ŪŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖX2O5£¬B”¢DĶ¬Ö÷×åĒŅBŌŖĖŲµÄŌ×Ó°ė¾¶ŹĒĶ¬×åŌŖĖŲÖŠ×īŠ”µÄ£¬CµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļŹĒĒæĖį”£
£Ø1£©DŌŖĖŲ»łĢ¬Ō×ÓµÄĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ____________________”£
£Ø2£©A”¢C”¢XČżÖÖŌŖĖŲŌ×ӵĵŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖ________________£ØÓĆĻąÓ¦µÄŌŖĖŲ·ūŗÅ×÷“š£©”£
£Ø3£©B”¢X”¢DĒā»ÆĪļµÄ·ŠµćÓÉøßµ½µĶµÄĖ³ŠņĪŖ_______________£ØÓĆĻąÓ¦µÄ»ÆѧŹ½×÷“š£©”£
£Ø4£©CŌŖĖŲµÄŌ×ÓæÉŠĪ³É¶ąÖÖĄė×Ó£¬ŹŌĶĘ²āĻĀĮŠĪ¢Į£µÄĮ¢Ģå¹¹ŠĶ£ØCĪŖ×ÖÄø£¬²»ŹĒĢ¼ŌŖĖŲ£©£ŗ
Ī¢Į£ | CO32- | CO42- |
Į¢Ģå¹¹ŠĶĆū³Ę | _______________ | _______________ |
£Ø5£©ŌŖĖŲBµÄŅ»ÖÖĒā»ÆĪļB2H4¾ßÓŠÖŲŅŖµÄÓĆĶ¾”£ÓŠ¹ŲB2H4µÄĖµ·ØÕżČ·µÄŹĒ_______”£
A£®B2H4·Ö×Ó¼äæÉŠĪ³ÉĒā¼ü B£®BŌ×ÓŹĒsp3ŌÓ»Æ
C£®B2H4·Ö×ÓÖŠŗ¬ÓŠ5øö¦Ņ¼üŗĶ1øö¦Š¼ü D£®B2H4¾§Ģå±äĪŖŅŗĢ¬Ź±ĘĘ»µ¹²¼Ū¼ü
£Ø6£©EŌŖĖŲŗĶDŌŖĖŲŌŚĶ¬Ņ»ÖÜĘŚ£¬ŹōÓŚVIII×壬¼Ū²ćÓŠČżøöµ„µē×Ó£¬E(OH)2ĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬ŌŚÅصÄĒæ¼īČÜŅŗÖŠæÉŠĪ³ÉE(OH)42-£¬Š“³öE(OH)2ĖįŹ½µēĄėµÄµēĄė·½³ĢŹ½___________________”£
£Ø7£©FŌŖĖŲ»łĢ¬Ō×ÓM²ćÉĻÓŠ5¶Ō³É¶Ōµē×Ó£¬FŠĪ³ÉµÄµ„ÖŹÓŠ¦Ä”¢¦Ć”¢¦ĮČżÖÖĶ¬ĖŲŅģŠĪĢå£¬ČżÖÖ¾§°ū£ØČēĶ¼ĖłŹ¾£©ÖŠFŌ×ÓµÄÅäĪ»ŹżÖ®±ČĪŖ___________£¬¦Ä”¢¦Ć”¢¦ĮČżÖÖ¾§°ūµÄ±ß³¤Ö®±ČĪŖ_____________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÖŲŅŖ»Æ¹¤ŌĮĻCuSO4µÄÖʱøĶ¾¾¶¼°ŠŌÖŹČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ
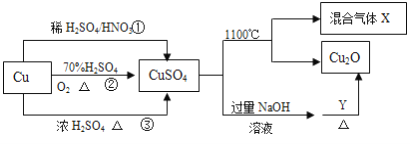
A. Ķ¾¾¶¢ŁĖłÓĆ»ģĖįÖŠH2SO4ÓėHNO3ĪļÖŹµÄĮæÖ®±Č×īŗĆĪŖ3£ŗ2
B. Ļą¶ŌÓŚĶ¾¾¶¢Ł”¢¢Ū£¬Ķ¾¾¶¢ŚøüŗƵŲĢåĻÖĮĖĀĢÉ«»ÆѧĖ¼Ļė
C. YĪļÖŹ¾ßÓŠ»¹ŌŠŌ£¬æÉŅŌŹĒĘĻĢŃĢĒ
D. 1molCuSO4ŌŚ1100”ęĖłµĆ»ģŗĻĘųĢåXÖŠO2Ņ»¶ØĪŖ0.75mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪļÖŹŹōÓŚµē½āÖŹµÄŹĒ
A. Š”ĖÕ“ņ B. ÕįĢĒ C. °±Ęų D. Ė®²£Į§
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com