
���� �����£�BΪ��ɫ��̬�л��B����Է�����Ϊ30����BΪC2H6��HCHO��X���Ҵ���ͬϵ�X+A��B+Y��B+C��X��X��YΪ������ɫҺ�壬A��CΪ��ɫ���壬����ƶ�AΪO2��BΪHCHO��XΪCH3OH��CH3OH������������HCHO��ˮ������YΪH2O��HCHO�����������ӳɷ�Ӧ��CH3OH������CΪH2��������������Ӧ����ˮ������A+C��Y���ݴ˴��⣮
��� �⣺�����£�BΪ��ɫ��̬�л��B����Է�����Ϊ30����BΪC2H6��HCHO��X���Ҵ���ͬϵ�X+A��B+Y��B+C��X��X��YΪ������ɫҺ�壬A��CΪ��ɫ���壬����ƶ�AΪO2��BΪHCHO��XΪCH3OH��CH3OH������������HCHO��ˮ������YΪH2O��HCHO�����������ӳɷ�Ӧ��CH3OH������CΪH2��������������Ӧ����ˮ������A+C��Y��
��������ķ�����֪��BΪHCHO��XΪCH3OH��X+A��B+Y�Ļ�ѧ����ʽΪ��CH3OH+O2$��_{��}^{����}$HCHO+H2O��B+C��X�Ļ�ѧ����ʽΪ��HCHO+H2$\stackrel{����}{��}$CH3OH��
�ʴ�Ϊ��HCHO��CH3OH��CH3OH+O2$��_{��}^{����}$HCHO+H2O��HCHO+H2$\stackrel{����}{��}$CH3OH��
���� ������Ҫ������Ԫ�ؼ��仯������ƶ������ʣ��е��Ѷȣ����ݸ����ʵ����Ӧ���������ʵ���Է������������ƶ��ǽ���Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ���� | ���� |
| A | �������ữ��Ba��NO3��2��Һ��ͨ��SO2 | �а�ɫ�������� | BaSO3�������� |
| B | ���Ũ�ȵ�KCl��Kl���Һ�еμ�AgNO3��Һ | �ȳ��ְ�ɫ���� | Ksp��AgCl����Ksp��AgI�� |
| C | ��������NaOHˮ��Һ���Ⱥ�HNO3�ữ���μ�AgNO3��Һ | ���ֵ���ɫ���� | �����麬��Ԫ�� |
| D | ȡ���õ�Na2O2��ĩ�������еμӹ��������� | ������ɫ���� | Na2O2û�б��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �е㣺H2O��H2Se��H2S | B�� | ���ԣ�H2SO4��H2CO3��HClO | ||
| C�� | Ӳ�ȣ�I2��Br2��Cl2 | D�� | ���ԣ�KOH��NaOH��Al��OH��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
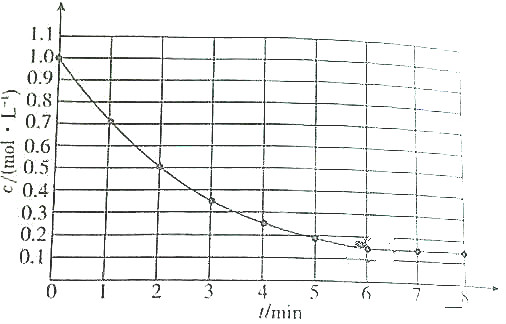
| A�� | ��6min������A��ƽ������Ϊ0mol/��L•min�� | |
| B�� | A��ƽ��ת����Ϊ85% | |
| C�� | �����¶�ʱ������A��ƽ�����ʴ�������A��ƽ������ | |
| D�� | �����¶�ʱ������A��ƽ�����ʺ�����A��ƽ�������Բ�ͬ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 2001������������þ������ˢ���˽��������ﳬ���¶ȵ����¼���û��Ͼ���ṹ�еľ�����ͼ��ʾ��þԭ�Ӽ��γ�����������������ԭ��λ�������ڣ�
2001������������þ������ˢ���˽��������ﳬ���¶ȵ����¼���û��Ͼ���ṹ�еľ�����ͼ��ʾ��þԭ�Ӽ��γ�����������������ԭ��λ�������ڣ� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɫ��Һ�п��ܴ�������Al3+��NH4+��Na+��Cl?��S2?��AlO2- | |
| B�� | c��H+����c��OH-��=1��1012����Һ��Mg2+��Cu2+��Fe2+��SO42-��Cl?��NO3-���Դ������� | |
| C�� | pH=8����Һ�п��ܴ�������Na+��K+��Ba2+��Cl-��HCO3-��NO3- | |
| D�� | ������Һ�п��ܴ�������Fe3+��K+��Al3+��Cl-��SO42-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7.1% | B�� | 35.5% | C�� | 28.4% | D�� | 42.6% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com