
 ʵŃéÖĆȡŇŇϩʱŁ¬Ó¦˝«ŇŇ´ĽşÍŨÁňËáżěËŮĽÓČȵ˝170ˇćŁ¬ÔÚ140ˇćʱ»áÉúłÉŇŇĂŃŁ¬Î¶ȹý¸ß»áĘą˛ż·ÖŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉSO2ˇ˘CO2ˇ˘Ë®ŐôĆřŁ®
ʵŃéÖĆȡŇŇϩʱŁ¬Ó¦˝«ŇŇ´ĽşÍŨÁňËáżěËŮĽÓČȵ˝170ˇćŁ¬ÔÚ140ˇćʱ»áÉúłÉŇŇĂŃŁ¬Î¶ȹý¸ß»áĘą˛ż·ÖŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉSO2ˇ˘CO2ˇ˘Ë®ŐôĆřŁ®| ŨÁňËá |
| 170ˇć |
| ŨÁňËá |
| 170ˇć |
| ŨÁňËá |
| 140ˇć |
| ŨÁňËá |
| 140ˇć |
| ¸ßΠ|
| ¸ßΠ|
| ŨÁňËá |
| 170ˇć |
| ŨÁňËá |
| 140ˇć |
| ¸ßΠ|
| ŨÁňËá |
| 170ˇć |
| ŨÁňËá |
| 140ˇć |
| ¸ßΠ|


 ĂűĐŁżÎĚĂϵÁĐ´đ°¸
ĂűĐŁżÎĚĂϵÁĐ´đ°¸
| Ä꼶 | ¸ßÖĐżÎłĚ | Ä꼶 | łőÖĐżÎłĚ |
| ¸ßŇ» | ¸ßŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ | łőŇ» | łőŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ß¶ţ | ¸ß¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ | łő¶ţ | łő¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ßČý | ¸ßČýĂâ·ŃżÎłĚÍĆĽöŁˇ | łőČý | łőČýĂâ·ŃżÎłĚÍĆĽöŁˇ |
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁş
ʵŃéÖĆȡŇŇϩʱŁ¬Ó¦˝«ŇŇ´ĽşÍŨÁňËáżěËŮĽÓČȵ˝170ˇćŁ¬ÔÚ140ˇćʱ»áÉúłÉŇŇĂŃŁ¬Î¶ȹý¸ß»áĘą˛ż·ÖŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉSO2ˇ˘CO2ˇ˘Ë®ŐôĆřˇŁ
Ł¨1Ł©·Ö±đĐ´łöŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉ˘ŮÍŃË®ÉúłÉCH2=CH2Ł»˘Ú·Ö×ÓĽäÍŃË®ÉúłÉCH3CH2-O-CH2CH3Ł»˘ŰSO2ˇ˘CO2ˇ˘Ë®ŐôĆřµÄ»ŻŃ§·˝łĚĘ˝
˘Ů Ł»
˘Ú Ł»
˘Ű Ł»
Ł¨2Ł©¶ţŃő»ŻÁňĘÇ´óĆřÎŰČľÎď֮һŁ¬żŐ
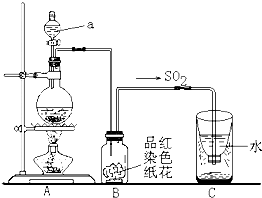
ĆřÖеĶţŃő»ŻÁňËćÓęË®Ď½µĐÎłÉËáÓꡣČçĎÂͼʵŃé×°ÖĂŁ¬¶ÔËáÓęµÄĐγɽřĐĐÄŁÄⲢŃéÖ¤¶ţŃő»ŻÁňµÄ˛ż·ÖĐÔÖĘŁ¬Çë»Ř´đŁş
˘ŮÖ¸łöÍĽÖĐŇÇĆ÷aµÄĂűłĆŁş ˇŁ
˘ÚB×°ÖĂÖеşěÉ«Ö˝»¨µÄŃŐÉ«Ö𽥱äÍĘČĄŁ¬ËµĂ÷SO2ÓĐ ĐÔˇŁ
˘Ű·´Ó¦˝áĘřşóȡłöC×°ÖĂÖĐÉŮÁżŇşĚ壬µÎĽÓ×ĎÉ«ĘŻČďĘÔŇşşó±ä É«ˇŁ
˘ÜʵŃéÍę±ĎşóˇŁÎŞÁËĘą¶ŕÓŕµÄ¶ţŃő»ŻÁň±»łä·ÖÎüĘŐŁ¬C×°ÖĂÓ¦ČçşÎ¸Ä˝řŁż
Ł¨ÓĂÎÄ×Ö˵Ă÷Ł© ˇŁ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş2011-2012ѧÄęąăÎ÷±±şŁşĎĆÖĎŘ˝ĚÓýľÖ¸ß¶ţĎÂѧĆÚĆÚÖĐÎĿƻŻŃ§ĘÔľíŁ¨´ř˝âÎöŁ© ĚâĐÍŁşĘµŃéĚâ
ʵŃéÖĆȡŇŇϩʱŁ¬Ó¦˝«ŇŇ´ĽşÍŨÁňËáżěËŮĽÓČȵ˝170ˇćŁ¬ÔÚ140ˇćʱ»áÉúłÉŇŇĂŃŁ¬Î¶ȹý¸ß»áĘą˛ż·ÖŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉSO2ˇ˘CO2ˇ˘Ë®ŐôĆřˇŁ
Ł¨1Ł©·Ö±đĐ´łöŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉ˘ŮÍŃË®ÉúłÉCH2=CH2Ł»˘Ú·Ö×ÓĽäÍŃË®ÉúłÉCH3CH2-O-CH2CH3Ł»˘ŰSO2ˇ˘CO2ˇ˘Ë®ŐôĆřµÄ»ŻŃ§·˝łĚĘ˝
˘Ů Ł»
˘Ú Ł»
˘Ű Ł»
Ł¨2Ł©¶ţŃő»ŻÁňĘÇ´óĆřÎŰČľÎď֮һŁ¬żŐ
ĆřÖеĶţŃő»ŻÁňËćÓęË®Ď½µĐÎłÉËáÓꡣČçĎÂͼʵŃé×°ÖĂŁ¬¶ÔËáÓęµÄĐγɽřĐĐÄŁÄⲢŃéÖ¤¶ţŃő»ŻÁňµÄ˛ż·ÖĐÔÖĘŁ¬Çë»Ř´đŁş
˘ŮÖ¸łöÍĽÖĐŇÇĆ÷aµÄĂűłĆŁş ˇŁ
˘ÚB×°ÖĂÖеşěÉ«Ö˝»¨µÄŃŐÉ«Ö𽥱äÍĘČĄŁ¬ËµĂ÷SO2ÓĐ ĐÔˇŁ
˘Ű·´Ó¦˝áĘřşóȡłöC×°ÖĂÖĐÉŮÁżŇşĚ壬µÎĽÓ×ĎÉ«ĘŻČďĘÔŇşşó±ä É«ˇŁ
˘ÜʵŃéÍę±ĎşóˇŁÎŞÁËĘą¶ŕÓŕµÄ¶ţŃő»ŻÁň±»łä·ÖÎüĘŐŁ¬C×°ÖĂÓ¦ČçşÎ¸Ä˝řŁż
Ł¨ÓĂÎÄ×Ö˵Ă÷Ł© ˇŁ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş2013˝ěąăÎ÷±±şŁşĎĆÖĎŘ˝ĚÓýľÖ¸ß¶ţĎÂѧĆÚĆÚÖĐÎĿƻŻŃ§ĘÔľíŁ¨˝âÎö°ćŁ© ĚâĐÍŁşĘµŃéĚâ
ʵŃéÖĆȡŇŇϩʱŁ¬Ó¦˝«ŇŇ´ĽşÍŨÁňËáżěËŮĽÓČȵ˝170ˇćŁ¬ÔÚ140ˇćʱ»áÉúłÉŇŇĂŃŁ¬Î¶ȹý¸ß»áĘą˛ż·ÖŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉSO2ˇ˘CO2ˇ˘Ë®ŐôĆřˇŁ
Ł¨1Ł©·Ö±đĐ´łöŇŇ´Ľ¸úŨH2SO4·´Ó¦ÉúłÉ˘ŮÍŃË®ÉúłÉCH2=CH2Ł»˘Ú·Ö×ÓĽäÍŃË®ÉúłÉCH3CH2-O-CH2CH3Ł»˘ŰSO2ˇ˘CO2ˇ˘Ë®ŐôĆřµÄ»ŻŃ§·˝łĚĘ˝
˘Ů Ł»
˘Ú Ł»
˘Ű Ł»
Ł¨2Ł©¶ţŃő»ŻÁňĘÇ´óĆřÎŰČľÎď֮һŁ¬żŐ
ĆřÖеĶţŃő»ŻÁňËćÓęË®Ď½µĐÎłÉËáÓꡣČçĎÂͼʵŃé×°ÖĂŁ¬¶ÔËáÓęµÄĐγɽřĐĐÄŁÄⲢŃéÖ¤¶ţŃő»ŻÁňµÄ˛ż·ÖĐÔÖĘŁ¬Çë»Ř´đŁş

˘ŮÖ¸łöÍĽÖĐŇÇĆ÷aµÄĂűłĆŁş ˇŁ
˘ÚB×°ÖĂÖеşěÉ«Ö˝»¨µÄŃŐÉ«Ö𽥱äÍĘČĄŁ¬ËµĂ÷SO2ÓĐ ĐÔˇŁ
˘Ű·´Ó¦˝áĘřşóȡłöC×°ÖĂÖĐÉŮÁżŇşĚ壬µÎĽÓ×ĎÉ«ĘŻČďĘÔŇşşó±ä É«ˇŁ
˘ÜʵŃéÍę±ĎşóˇŁÎŞÁËĘą¶ŕÓŕµÄ¶ţŃő»ŻÁň±»łä·ÖÎüĘŐŁ¬C×°ÖĂÓ¦ČçşÎ¸Ä˝řŁż
Ł¨ÓĂÎÄ×Ö˵Ă÷Ł© ˇŁ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş2011-2012ѧÄęąăÎ÷±±şŁĘĐşĎĆÖĎظ߶ţŁ¨ĎÂŁ©ĆÚÖĐ»ŻŃ§ĘÔľíŁ¨ÎĿƣ©Ł¨˝âÎö°ćŁ© ĚâĐÍŁş˝â´đĚâ

˛éż´´đ°¸şÍ˝âÎö>>
°Ů¶ČÖÂĐĹ - Á·Ď°˛áÁбí - ĘÔĚâÁбí
şţ±±Ęˇ»ĄÁŞÍřÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨Ć˝Ě¨ | ÍřÉĎÓĐş¦ĐĹϢľŮ±¨×¨Çř | µçĐĹթƾٱ¨×¨Çř | ÉćŔúĘ·ĐéÎŢÖ÷ŇĺÓĐş¦ĐĹϢľŮ±¨×¨Çř | ÉćĆóÇÖȨľŮ±¨×¨Çř
ÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨µç»°Łş027-86699610 ľŮ±¨ÓĘĎ䣺58377363@163.com