
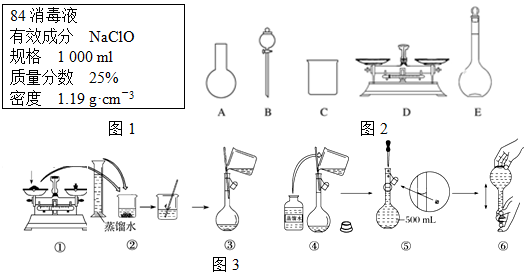
���� ��1�����ݺ�25%NaClO��1000mL���ܶ�1.19g•cm-3�����c=$\frac{1000��w}{M}$������������Ƶ����ʵ���Ũ�ȣ�����ϡ��ǰ�����ʵ����ʵ������������㣻
��2��������Һ�����Ƽ�c=$\frac{n}{V}$��m=nM��������
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ݴ˽��
��4��������Һϡ��ǰ�����ʵ����ʵ���������㣮
��� �⣺��1��c��NaClO��=c=$\frac{1000��w}{M}$=$\frac{1000��1.19��25%}{74.5}$=4.0 mol•L-1��ϡ�ͺ�c��NaClO��=$\frac{1}{100}$��4.0 mol•L-1=0.04 mol•L-1��c��Na+��=c��NaClO��=0.04 mol•L-1��
�ʴ�Ϊ��0.04��
��2��A������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ��A��B������Ҫ�������貣�����ͽ�ͷ�ιܣ���A����
B�����ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã���B����
C���ƹ����У�δ������ˮϴ���ձ��Ͳ��������������Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�ͣ���C��ȷ��
D��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO��������0.5 L��4.0 mol•L-1��74.5 g•mol-1=149 g����D����
�ʴ�Ϊ��C��
��3����Һ��δϴ���ձ��Ͳ�����������ʱ����Ӧƽ�ӿ̶��ߣ�
��ѡ��B��
��4����Һϡ��ǰ�����ʵ����ʵ������䣬Ũ�����Ũ��Ϊc=$\frac{1000��1.84g/L��98%}{98g/mol}$=18.4mol/L��������ҪŨ��������ΪV��
��V��18.4mol/L=2L��2.3mol/L��V=0.25L=250mL��
�ʴ�Ϊ��250��
���� ���⿼�����ʵ���Ũ�ȵļ����Լ���Һ�����ƣ�Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬ע�������ؼ��㹫ʽ�����ã��ѶȲ���


 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3-������ | B�� | 2��2��4��4-�ļ����� | ||
| C�� | 2-��-3-��ϩ������ | D�� | 2-��-3-��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ������ᣩ ��ʯ�� ���� | |
| B�� | �Ҵ������ӣ� NaOH��Һ ��Һ | |
| C�� | �����飨�Ҵ��� ˮ ��Һ | |
| D�� | �����ᣨ�������ƣ����� ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1molNa2O2������ˮ��Ӧת�Ƶ�����Ϊ2NA | |
| B�� | 28g N2��CO�Ļ�������к���ԭ�ӵ���Ŀ����2NA | |
| C�� | ���³�ѹ�£�22.4L NH3���ӵĵ���������������Ϊ10NA | |
| D�� | 1mol N2��3mol H2��ϣ���ַ�Ӧ�����ɰ���������Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������2-�����飻
��������2-�����飻 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����������
���������� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ���и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
���и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������| a | b | c | |
| A | Al | AlCl3 | Al��OH��3 |
| B | NO | NO2 | HNO3 |
| C | Si | SiO2 | H2SiO3 |
| D | FeS2 | SO2 | H2SO4 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ˮ��Fe3+��NH4+��SO42-��OH- | |
| B�� | �������NaOH��Һ��Na+��AlO2-��SO42-��OH- | |
| C�� | �������H2O2��Һ��Fe2+��H+��SO42-��Cu2+ | |
| D�� | �������NaHCO3��Һ��Na+��Al3+��SO42-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com