
��8�֣�ij����С�飬�ú���������CaO��Fe2O3�ĸ���������Ҫ�ɷ֣�Al2O3��2SiO2��2H2O�����������;�ˮ������ʵ�鷽�����£��������ʹ����Ͼ��ȣ��������ڣ���ȴ����ˮ��ȡ�ۿ飬������ȥ��������Һ�������ữ�������ˣ��ֱ�õ���������Һ����Һ���Ǿ�ˮ����
��1�������ʹ����Ͼ��ȣ���������ʱ�������е���Ҫ�ɷ��봿�Ӧ�Ļ�ѧ����ʽΪ��
��
����Al2O3��Na2CO3 2NaAlO2��CO2����
2NaAlO2��CO2����
����ʾ��NaAlO2������ˮ��ת��ΪNa[Al (OH)4]��
��2�����õ��ij������� �����ɳ��������ӷ���ʽΪ ��
��3��ʵ�����г����������д�����������������������������ʵ����������ʱӦѡ�� ������
��1��SiO2��Na2CO3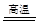 Na2SiO3��CO2����2��H4SiO4��2H����SiO32����H2O��H4SiO4����3����
Na2SiO3��CO2����2��H4SiO4��2H����SiO32����H2O��H4SiO4����3����
������������һ����Ϣ�����⣬������ʾ�龳��������1���������ۻ�ʱ����ɷ��е�SiO2��Al2O3��Na2CO3��Ӧ����Na2SiO3��NaAlO2����2���ۿ��ˮʹNa2SiO3��NaAlO2�ܽ����������ʷ��룬�ڹ��˺����Һ�м���������ʹNa2SiO3��Na[Al (OH)4]�ֱ�����������ᷴӦ������H4SiO4������AlCl3��Һ�����˵���Һ��Ϊ��ˮ������3����Ϊ�������к���SiO2��������������Ҫ�ɷ���Al2O3�����Ƕ�����Na2CO3�ڸ����·�����Ӧ����������Ʒʱֻ������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���ܽ�ʱ����Ҫ�ɷ��봿�Ӧ�Ļ�ѧ����ʽΪ����ʾ����Al2O3�봿��ķ�Ӧ��SiO2�봿��ķ�Ӧ���ƣ���NaAlO2����ˮ����
��_______________________________________________________________��
��_______________________________________________________________��
��2�����õ��ij�������____________�����ɳ��������ӷ���ʽΪ____________________��
��3��ʵ�����г����������д�����������������������������ʵ����������ʱӦѡ��_____________������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С�飬�ú���������CaO��Fe2O3�ĸ���������Ҫ�ɷ֣�Al2O3��2SiO2��2H2O�����������;�ˮ������ʵ�鷽�����£��������ʹ����Ͼ��ȣ��������ڣ���ȴ����ˮ��ȡ�ۿ飬������ȥ��������Һ�������ữ�������ˣ��ֱ�õ���������Һ����Һ���Ǿ�ˮ����
��1�������ʹ����Ͼ��ȣ���������ʱ�������е���Ҫ�ɷ��봿�Ӧ�Ļ�ѧ����ʽΪ��
�� ����Al2O3��Na2CO3![]() 2NaAlO2��CO2����
2NaAlO2��CO2����
����ʾ��NaAlO2������ˮ��ת��ΪNa[Al (OH)4]��
��2�����õ��ij������� �����ɳ��������ӷ���ʽΪ ��
��3��ʵ�����г����������д�����������������������������ʵ����������ʱӦѡ�� ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�ij����С�飬�ú���������CaO��Fe2O3�ĸ���������Ҫ�ɷ֣�Al2O3��2SiO2��2H2O�����������;�ˮ������ʵ�鷽�����£��������ʹ����Ͼ��ȣ��������ڣ���ȴ����ˮ��ȡ�ۿ飬������ȥ��������Һ�������ữ�������ˣ��ֱ�õ���������Һ����Һ���Ǿ�ˮ����
��1�������ʹ����Ͼ��ȣ���������ʱ�������е���Ҫ�ɷ��봿�Ӧ�Ļ�ѧ����ʽΪ��
�� ����Al2O3��Na2CO3![]() 2NaAlO2��CO2����
2NaAlO2��CO2����
����ʾ��NaAlO2������ˮ��ת��ΪNa[Al (OH)4]��
��2�����õ��ij������� �����ɳ��������ӷ���ʽΪ ��
��3��ʵ�����г����������д�����������������������������ʵ����������ʱӦѡ�� ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³�����1��04�µ�Ԫ���� ���ͣ�ʵ����
��8�֣�ij����С�飬�ú���������CaO��Fe2O3�ĸ���������Ҫ�ɷ֣�Al2O3��2SiO2��2H2O�����������;�ˮ������ʵ�鷽�����£��������ʹ����Ͼ��ȣ��������ڣ���ȴ����ˮ��ȡ�ۿ飬������ȥ��������Һ�������ữ�������ˣ��ֱ�õ���������Һ����Һ���Ǿ�ˮ����
��1�������ʹ����Ͼ��ȣ���������ʱ�������е���Ҫ�ɷ��봿�Ӧ�Ļ�ѧ����ʽΪ��
�� ����Al2O3��Na2CO3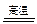 2NaAlO2��CO2����
2NaAlO2��CO2����
����ʾ��NaAlO2������ˮ��ת��ΪNa[Al (OH)4]��
��2�����õ��ij������� �����ɳ��������ӷ���ʽΪ ��
��3��ʵ�����г����������д�����������������������������ʵ����������ʱӦѡ�� ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com