
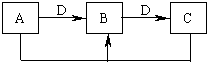
��9�֣���һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش��������⣬
��֪A��B��C�о�����ͬһ��Ԫ�أ�DΪ�ǽ������ʣ���ʹ���л��ǵ�ľ����ȼ��
��1����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��д��A���ʵĵ���ʽ ��
��C��ˮ��Ӧ���õ���Һ�����ԣ��˷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ����
��2����AΪ���嵥�ʣ�CΪ����ɫ���壬����C���еĻ�ѧ���� ��
A��ˮ��Ӧ�����ӷ���ʽΪ ��
��3����AΪ�ճ�����������������������A��C��Ӧ����һ����ˮ����ζ�����ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ , ��Ӧ����Ϊ ��Ӧ��
 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش��������⣬��֪A��B��C�о�����ͬһ��Ԫ�أ�DΪ�ǽ������ʣ���ʹ���л��ǵ�ľ����ȼ��
��һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش��������⣬��֪A��B��C�о�����ͬһ��Ԫ�أ�DΪ�ǽ������ʣ���ʹ���л��ǵ�ľ����ȼ��| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2SO2 (g)+O2 (g) ![]() 2SO3(g)����H=-196.64 kJ��mol�����ų�314.624 kJ����ʱ��SO2��ת����Ϊ( )
2SO3(g)����H=-196.64 kJ��mol�����ų�314.624 kJ����ʱ��SO2��ת����Ϊ( )
A.40�� B.50�� C.80�� D.90��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣���һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش��������⣬
��֪A��B��C�о�����ͬһ��Ԫ�أ�DΪ�ǽ������ʣ���ʹ���л��ǵ�ľ����ȼ��
��1����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��д��A���ʵĵ���ʽ ��
��C��ˮ��Ӧ���õ���Һ�����ԣ��˷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ����
��2����AΪ���嵥�ʣ�CΪ����ɫ���壬����C���еĻ�ѧ���� ��
A��ˮ��Ӧ�����ӷ���ʽΪ ��
��3����AΪ�ճ�����������������������A��C��Ӧ����һ����ˮ����ζ�����ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ , ��Ӧ����Ϊ ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ʵ������4molSO2��2molO2��һ�������½������з�Ӧ��
2SO2��g��+O2��g�� 2SO3��g������H=��197kJ/mol�����ų�315.2kJ����ʱ��SO2��ת����Ϊ
2SO3��g������H=��197kJ/mol�����ų�315.2kJ����ʱ��SO2��ת����Ϊ
A��40% B��50% C��80% D��90%
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com