
��14�֣�ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽����
a������ƿ��ע��������NaOH��Һ����ʢ��һ�������Ĵ����۵�С�ձ�����ƿ�С�
b���ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c�������׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��л�������һ������4mol��L��1��ϡ���ᣬ
������ȫ�ܽ⡣
��һ���������ɷ�̽��
��1��ʵ��ǰ��μ���װ�õ������ԣ� ��
��2��ȼ�պ���Ŀ���� ��
��3��Ϊ֤���������ΪNO������c��ȱ�ٵ�һ����Ҫ������ ��
��������������Ԫ�ؼ�̬̽����
��1������������裺
����1��������ֻ��+2����������2�� ������3�������м���+2����������+3������
��2�����ʵ�鷽������֤����1������д��ʵ��������衢����ͽ��ۣ��� ��
��������������
��1�����ƿ����NaOH��Һ����Ҫ������ ��
��2��������3��������������Һ��n(Fe2+)��n(Fe3+)=3��1ʱ�����Ӧ�����ӷ�Ӧ����ʽΪ ��
��һ����1���ر�ֹˮ�У�ͨ����Һ©������ƿ�м�ˮ����ˮ����˳������ʱֹͣ��ˮ���۲��Һ©����ˮ�棬��ˮ�治�½���˵��װ�����������ã���2����ȥ���ƿ�ڿ����е��������������������飻��3����ֹˮ�У���ƿ��Ǩ����������������������
��������1��������ֻ��+3��������2��ȡС�ձ��з�Ӧ����Һ����������һֻ�Թ��У��μ�KSCN��Һ����Һ���Ժ�ɫ���������еμ�������H2O2��Һ����Һ�Ժ�ɫ����֤������1������
��������1��ʵ���������NaOH��Һ�ڳ����������������������������£���ƿ�е�����������ȫ���գ��Է�ֹ������Ⱦ����2��4Fe+12H++3NO3-=3Fe2++Fe3++3NO��+5H2O
����


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
2012�궬�죬�ҹ����п�����Ⱦ״���ܵ����ǵ�ǿ�ҹ�ע���ڿ������������У�SO2��ָ���Ǻ������������û�����Ҫָ�ꡣΪ�˲ⶨ�����е�SO2����������λͬѧ�ֱ�������������ֲⶨ������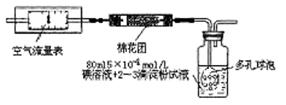
I��������ԭ������ͬѧ���ݻ�ѧ��Ӧԭ��SO2+I2+2H2O=H2SO4+2HI���������ͼ��ʾ��װ�ý���ʵ�飺
��1����ʵ����80mlŨ��Ϊ5��10-4mol/L�ĵ�
��Һ����ͬѧӦѡ�� ml������ƿ�������ơ�
��2�����ƿ��ʹ�ö�����ݵ�Ŀ���� ��
��3���ڼ�ͬѧ�������ҺŨ��ȷ��������ȡҩƷ�� ʵ������и��ֶ��������������£���������װ�����ⶨ��SO2������Ȼ��ʵ�ʺ����ͣ���������п��ܵ�ԭ������д����ԭ��
II������������ͬѧ����ʵ���ҳ���������ɼ���װ�òⶨ�����е�SO2������ʵ��������£�
������ʵ��װ��ͼ��װ���������ڹ��ƿ��ʢ��������H2O2ˮ��Һ���ù��Ϊ20ml����Ͳ����100�Σ�ʹ�����е�SO2��H2O2ˮ��Һ������գ�SO2+H2O2=H2SO4���������պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ������H2SO4+BaCl2=BaSO4��+2HCl���������ˡ�ϴ�ӡ�����Ȳ������г������ð�ɫ����0��18mg��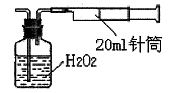
��4��ȡ����������SO2����Ϊ mg/L����ȷ��0.001����
��5���ֲ�������֪��������BaSO3��KspΪ5��48��10-7��������������Һ��c(SO32-)=6.3��10-8mol/L����ͬѧ��Ϊ����ʵ�鲻����H2O2����SO2��ֱ����0.lmol/L BaCl2��Һ������SO2���ɲ�������������Ϊ������ �����ȷ������ȷ�������������������ݼ������� ��
III������������ͬѧֱ��ʹ��һ��SO2Ũ�����ܼ���Dzⶨ�����е�SO2���������ּ���������õ绯ѧԭ�������ݵ�ز���������ǿ����ȷ����SO2Ũ�ȵġ��õ���ܵĻ�ѧ��Ӧԭ��Ϊ��2SO2+O2+2H2O=2H2SO4��
��6����д���õ�ظ����ĵ缫��Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
������þ(MgO2)������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȡ�������þ��Ʒ�г����������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ����� 
��1��ij�о�С��������ͼװ�òⶨһ����������Ʒ�й�����þ�ĺ�����
��ʵ��ǰ����еIJ����� ��ϡ�����м�������FeCl3��Һ�������� ��
���ú�ѹ��Һ©�����ŵ��У�ʹ��Һ©���е���Һ˳�����£� ��
��ʵ������ʱ�����ָ������£��� ����ƽ�ӿ̶��߶�����
��2��ʵ���һ���ͨ���������ַ����ⶨ��Ʒ�й�����þ�ĺ�����
����I��ȡa g��Ʒ����������ϡ���ᣬ��ַ�Ӧ���ټ��� NaOH��Һ��Mg2��������ȫ�����ˡ�ϴ�Ӻ�����������գ����յõ�b g���塣
������ȡ0.1 g��Ʒ���ڵ���ƿ�У�����15 mL0.6 mol/LKI��Һ���������ᣬҡ�Ⱥ��ڰ�������5 min��Ȼ����0.1 mol/L Na2S2O3��Һ�ζ����ζ����յ�ʱ������VmL Na2S2O3��Һ��(��֪��I2+2Na2S2O3= Na2S4O6+2NaI)
����֪������Ksp[Mg(OH)2]=l��10��11��Ϊʹ����I��Mg2+��ȫ����[����Һ��c(Mg2+)��l ��10��5mol/L]����Һ��pH����Ӧ���� ������I�й�����þ����������Ϊ (�ú�a��b�ı���ʽ��ʾ)��
�ڷ������еζ�ǰ��������� ��ָʾ������Ʒ�й�����þ����������Ϊ ���ú�V�ı���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��20�֣���ҵ�ϳ����ú����ˮ����Na2S2O3?5H2O��ʵ���ҿ�������װ�ã���ȥ���ּӳ�������ģ�����ɹ��̡�
��ƿC�з�����Ӧ���£�
Na2S��aq��+H2O��l��+SO2��g��=Na2SO3��aq��+H2S��aq�� ��I��
2H2S��aq��+SO2��g��=3S��s��+2H2O��l�� ��II��
S��s��+Na2SO3��aq�� Na2S2O3��aq�� ��III��
Na2S2O3��aq�� ��III��
��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һע���� ��������װ�����������á�װ��D��������
��װ��E��Ϊ ��Һ��
��2��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ ��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ�� ��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ����� ����֪��Ӧ��III����Խ���������ƿC�з�Ӧ�ﵽ�յ�������� ����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ�������������� ��
a���ձ� b�������� c���Թ� d����ƿ
��4����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O3?5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʡ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�
��
��֪Na2S2O3?5H2O�����ֽ⣺S2O32?+2H+=S��+SO2��+H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������(NH2COONH4)��һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȡ�ij��ѧ��ȤС��ģ�ҵԭ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�2 NH3(g)+CO2(g)  NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0 
��1��ʵ�����Ʊ�NH3�Ļ�ѧ����ʽ�ǣ� ��
��2���Ʊ���������淋�װ������ͼ��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�С���������϶�ʱ��ֹͣ�Ʊ���
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʡ�
�ٷ������ñ�ˮ��ȴ��ԭ���� ��Һ��ʯ������ƿ�������� ��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���� (��д��������)��Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ����� (��дѡ�����)��
a. ��ѹ���Ⱥ�� b. ��ѹ���Ⱥ�� c. ���40 �����º��
��β������װ������ͼ��ʾ��˫ͨ�����ܵ����ã� ��
Ũ��������ã� �� �� ����������������
��3��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7825 g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000 g������Ʒ�а�������淋����ʵ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�ʵ���ҴӺ����Һ����H2O�⣬����CCl4��I2��I���ȣ��л��յ⣬��ʵ��������£�
��1�����Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ ���ò�����I2��ԭΪI����Ŀ���� ��
��2������X������Ϊ ��
��3������ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����400C���ҷ�Ӧ��ʵ��װ����ͼ��ʾ����ʵ������ڽϵ��¶��½��е�ԭ���� ����ƿ��ʢ�ŵ���ҺΪ ��
��4����֪��5SO32��+2IO3��+2H�� I2+5SO42��+H2O
I2+5SO42��+H2O
ij�����ˮ��pHԼΪ8����һ������I2�����ܴ���I����IO3���е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO3����ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڣ� ��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����Ӫ���ḻ������������������š����˲����붹��ͬʳ����˵����ijѧУ��ѧ��ȤС���ͬѧ��ͨ��ʵ��̽���������Ƿ��в��������ʣ�����������Щ���ʣ�ͨ��������ѯ������������ϣ����������Ҷ��ᣬ�����Ա�������ǿ�����ἰ���ξ��н�ǿ�Ļ�ԭ�ԣ����ᾧ��(H2C2O4��2H2O)���۵�Ϊ100.1�棬��175��ʱ���ȷֽ⣬�������������ˮ�İ�ɫ���塣
������Ƶ�ʵ�鲽�����£�
1����������������ˮ�����2��3 min����ȴ����ȥ���ˣ�����Һ(�������� ����)������Һ�м�������Ca(OH)2��Һ��Ȼ���ټ�������CH3COOH��Һ���۲�����
����)������Һ�м�������Ca(OH)2��Һ��Ȼ���ټ�������CH3COOH��Һ���۲�����
2���ò��ᾧ��(H2C2O4��2H2O)������ʵ�飺
��ش��������⣺
(1)����1�м���CH3COOH��Һ�����ã� ��
(2)A��Ӧѡ�� (���)������ʵ��֮ǰ��Ӧ�� ��
(3)ʵ��2�����й۲쵽C��Eװ���е���Һ������ǣ���Dװ���к�ɫ��ĩ��Ϊ��ɫ��д��A�в��ᾧ��(H2C2O4��2H2O)������Ӧ�Ļ�ѧ����ʽ�� ��װ��B�������� ��
(4)Ϊʹʵ����۸������ܺͰ�ȫ�������������ӵ�װ��C��D�仹����������װ�� �� �� (Һ���Լ�)��ϴ��ƿ������ָ������װ���еIJ���֮������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʽ̼����A������θҩ������ɿɱ�ʾΪAl2Mg6(OH)x(CO3)y��zH2O��ijУ��ѧ��ȤС�����ⶨ�仯ѧʽ��ʵ��������£�
ʵ��I����ȡһ��������A�����ȷֽ������ء�
ʵ���ȡһ��������A���������ᷴӦ����������CO2�����������
�ɹ�ѡ���������ҩƷ��ͼ��ʾ��������Һ��ѡ6mol/LHCl��6mol/LH2SO4�������Լ���ѡ����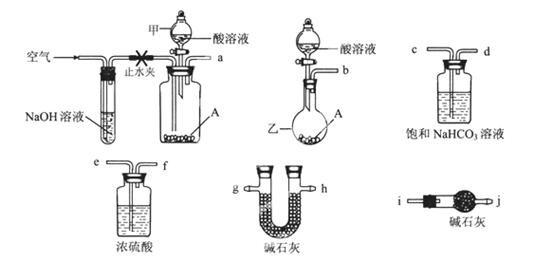
�ش��������⣺
(1)�����ҵ�����Ϊ________��
(2)��ѡ���Ҫ��װ�����ʵ��II,��ȷ������˳��Ϊ________ (�����������ýӿ���ĸ��ʾ����ѡ�õ�����Һ��________��
(3)�������������ʵ��I������ʵ��II��������A��ȫ��Ӧ��������Һ�еμ������İ�ˮ��������ֽ���ˣ�������ˮϴ�ӷ�Ӧ����2?3�Σ���ϴ��Һ���ˣ�ϴ�ӳ���2?3�Σ������ų�������ֽ�ŵ������м��ȷֽ������ء��жϳ�����ϴ�Ӹɾ��ķ�����_________________,ʵ����δ���ø÷�����ԭ���Dz�����ʵ����Ƶ�________ԭ������ĸ��ţ���
| A����ѧ�� | B����ȫ�� | C�������� | D����Լ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1�����һ����һ�������ʵ���װ�ã���֤���ᡢ������̼ˮ��Һ��̼�ᣩ�ͱ��ӵ����ԣ���ǿ����˳���ǣ�CH3COOH> H2CO3> C6H5OH
��������������������װʵ��װ�ã�������������˳���ǣ� ��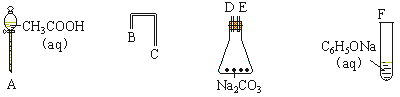
��д��ʵ������з�����Ӧ��ʵ������ �� ��
��2��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=2.8��10��9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2��10��4mo1/L �������ɳ�������CaCl2��Һ����СŨ��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com