
 ��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��Ҫ��д��298K��101kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ������ ��1��3molNO2��Ӧ�ų�138kJ���������H=-138 kJ/mol��ע���ʱ�����ʵ����Ķ�Ӧ��ϵ��
��2���ٷ�Ӧ�������������������������˷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ�������������������������÷�Ӧ�����ȷ�Ӧ��
��ȼ���ȵ���ָ��ȫȼ��1mol���������ȶ��������ų��������������Ȼ�ѧ����ʽ����д�������ش�
��3��������д�Ȼ�ѧ����ʽ�ķ�����������
��� �⣺��1��3molNO2��Ӧ�ų�138kJ��������H=-138 kJ/mol�����Ȼ�ѧ����ʽΪ3NO2��g��+H2O��l��=2HNO3��aq��+NO��g����H=-138 kJ/mol��
�ʴ�Ϊ��3NO2��g��+H2O��l��=2HNO3��aq��+NO��g����H=-138 kJ/mol��
��2���ٸ���ͼ����Ϣ����Ӧ�������������������������˷�Ӧ�Ƿ��ȷ�Ӧ�������ʱ��Ǹ�ֵ���ʴ�Ϊ��-��
��ȼ���ȵ���ָ��ȫȼ��1mol���������ȶ����������̼�����Һ̬ˮ���ų���������ͼ����һ����������ȫȼ������CO2��1mol H2O��l�������������Ա�����ȫȼ������CO2��4mol H2O��l��������Ϊ��553.75kJ/mol��4=2215.0 kJ/mol������C3H8��g��+5O2��g���T3CO2��g��+4H2O��l����H=-2215.0 kJ/mol��
�ʴ�Ϊ��C3H8��g��+5O2��g���T3CO2��g��+4H2O��l����H=-2215.0 kJ/mol��
��3��6g ̿���������в���ȫȼ������һ����̼���ų�55.2kJ��������1mol̿���������в���ȫȼ������һ����̼���ų�110.4kJ�������Ȼ�ѧ����ʽΪ��C��s��+$\frac{1}{2}$O2 ��g��=CO��g����H=-110.4 kJ•mol-1���ʴ�Ϊ��C��s��+$\frac{1}{2}$O2 ��g��=CO��g����H=-110.4 kJ•mol-1��
���� ���⿼��ѧ����Ӧ�������ȵ��ж��Լ��Ȼ�ѧ����ʽ����д��֪ʶ�������ۺ�֪ʶ�Ŀ��飬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij100mL��Һ�к��еIJ�������Ũ�ȴ�С��ͼ��ʾ������Һ���ܻ�����Fe3+��Ba2+��K+��OH-��NO3-��CO32-��SO42-��Ϊ�˽�һ��ȷ�ϣ��Ը���Һ����ʵ���⣺
ij100mL��Һ�к��еIJ�������Ũ�ȴ�С��ͼ��ʾ������Һ���ܻ�����Fe3+��Ba2+��K+��OH-��NO3-��CO32-��SO42-��Ϊ�˽�һ��ȷ�ϣ��Ը���Һ����ʵ���⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
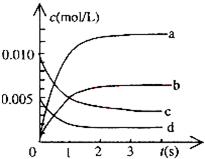 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
| ���� | HNO2 | HClO | H2CO3 | H2SO3 |
| ����ƽ�ⳣ�� ��25�棩 | Ki=5.1��10-4 | Ki=2.98��10-8 | $\begin{array}{l}{K_{i1}}=4.3��{10^{-7}}\\{K_{i2}}=5.6��{10^{-11}}\end{array}$ | $\begin{array}{l}{K_{i1}}=1.54��{10^{-2}}\\{K_{i2}}=1.02��{10^{-7}}\end{array}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȡ� | K1 | K2 |
| 500 | 1.00 | 3.15 |
| 700 | 1.47 | 2.26 |
| 900 | 2.40 | 1.60 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼�����
��ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷ�Ϊ���¼������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ������ˮ | |
| B�� | H2��ԭCuOʱ����ͨH2�����CuO����Ӧ��Ϻ���ֹͣͨH2�ƾ��� | |
| C�� | �����������ʱ���������е�����ˮӦ���¿ڽ����Ͽڳ� | |
| D�� | ��ҹ������ú��й©ʱ���ȴƣ���Ѹ�ٿ���ͨ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com