
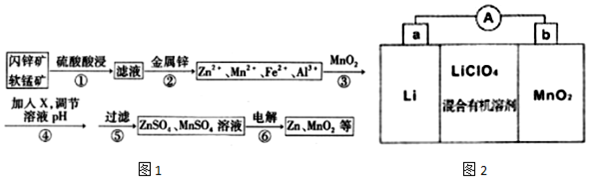
| Zn2+ | Mn2+ | Fe2+ | Fe3+ | Al3+ | |
| pH | 8.0 | 10.1 | 9.0 | 3.2 | 4.7 |
���� ���̿���п����Ҫ�ɷ�ΪMnO2��ZnS��������������FeS��CuS��CdS��Al2O3��SiO2�����ʣ��������ᣬ����3MnO2+2FeS+6H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$3MnSO4+Fe2��SO4��3+2S+6H2O���ú���Al3+��Mn 2+��Fe3+��Zn2+��Fe2+��Cu2+��Cd 2+��������Һ�������м���������п�ۣ���ͭ�����û���������ͭ��ͬʱ�������ӻ�ԭΪ�������ӣ�������Һ��Mn 2+��Zn2+��Fe2+��Al3+���м���MnO2���������������������ӣ�����MnCO3 ��Zn��OH��2����pH4.7��8.0��ʹ�����Ӻ������ӳ��������˵���Һ�к���MnSO4��ZnSO4�������Һ��п�Ͷ������̣��ݴ˴��⣮
��� �⣺��1��MnO2��FeS�����Ṳ��ʱ�е���ɫ��������������S����Һ��Ϊ�ػ�ɫ�������������ɣ��ʷ�Ӧ��3MnO2+2FeS+6H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$3MnSO4+Fe2��SO4��3+2S+6H2O��
�ʴ�Ϊ��3MnO2+2FeS+6H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$3MnSO4+Fe2��SO4��3+2S+6H2O��
��2�������ΪAl3+��Mn 2+��Fe3+��Zn2+��Fe2+��Cu2+��Cd 2+��������Һ���������п�õ�����Һ����Mn 2+��Zn2+��Fe2+��Al3+����п�۽�Cu2+��Cd 2+��ԭΪCu��Cd��
�ʴ�Ϊ��Cu��Cd��
��3���������MnO2�����������ӵķ�Ӧ�����ӷ���ʽMnO2+2Fe2++4H+=2Fe3++Mn 2++2H2O������X����pH4.7��8.0��ʹ�����Ӻ������ӳ��������˵���Һ�к���MnSO4��ZnSO4����XΪMnCO3 ��Zn��OH��2��
�ʴ�Ϊ��MnO2+2Fe2++4H+=2Fe3++Mn 2++2H2O��BD��
��4�����MnSO4��ZnSO4��Һ�ɵ�H2SO4��ѭ��ʹ�ã�
�ʴ�Ϊ��H2SO4��
��5����LiΪ������MnO2Ϊ������ԭ��ع���ʱ�������ƶ���Ӹ���������������a������b����
�ʴ�Ϊ��a��b��
��MnO2Ϊ����������ԭ���缫����ʽΪMnO2+e-+Li+=LiMnO2��
�ʴ�Ϊ��MnMnO2+e-+Li+=LiMnO2��
��6����֪��25��ʱ��HCN�ĵ��볣��K=4.9��10-10��H2S�ĵ��볣��K1=1.3��10-7��K2=7.0��10-15��������H2S��HCN��HS-������ǿ���Ʊ����ᣬ��NaCN��Һ��ͨ��������H2S���壬��NaCN+H2S=HCN+NaHS��
�ʴ�Ϊ��NaCN+H2S=HCN+NaHS��
���� ���⿼��ѧ����Ԫ�ؼ��仯�������Ҫ���ʵ����ա���д�缫��Ӧ����ʽ���Ķ���Ŀ��ȡ����Ϣ�������Թ������̵�����ȣ��Ѷ��еȣ���Ҫѧ���߱���ʵ�Ļ������ۺ�����֪ʶ����Ϣ�����������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��ʵ | ���۽��� |
| A | Na ��ˮ��Ӧʧȥ 1 �����ӣ�Mg ��ˮ��Ӧʧȥ 2 ������ | Na �Ľ����Ա� Mg ǿ |
| B | H2S �Ļ�ԭ�Ա� HCl | S �ķǽ����Ա� Cl |
| C | K3C60������״̬���ܹ����� | K3C60�к������� |
| D | Si �ǰ뵼����ϣ�ͬ����� Ge Ҳ �ǰ뵼����� | ��A Ԫ���γɵĵ��ʶ��ǰ뵼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
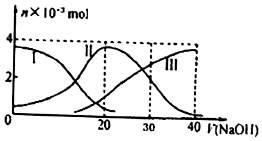
| A�� | H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A�TH++HA-��HA-?H++A2- | |
| B�� | �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� | |
| C�� | ��V��NaOH��=30 mLʱ����Һ�д������¹�ϵ��2c��H+ ��+c��HA- ��+2c��H2A���Tc��A2- ��+2c��OH-�� | |
| D�� | ��V��NaOH��=20 mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊc��Na+����c��HA-����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ���Ũ��֮��Ϊc��A����c��B����c��C��=2��3��3 | |
| B�� | ���淴Ӧ��������ҵ����� | |
| C�� | ƽ�������и����ʵ���Ũ����� | |
| D�� | ��λʱ���ڣ���������amolA���ʣ���ͬʱҲ������1.5amolC���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ�ĵ�������һ���Ȱ�ˮǿ | |
| B�� | �к͵�����������ʵ���Ũ�ȵ�����ʹ��ᣬ��Ҫ������NaOH | |
| C�� | �������Ũ���Ǵ���Ũ�ȵ��������������c��H+��Ҳ�Ǵ���c��H+�������� | |
| D�� | ��NaOH��Һ�Ͱ�ˮ��ϡ��һ�������ߵ�c��OH-������С��ԭ����һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ǽ�����P��S | |
| B�� | Na3PO4��Һ�ʼ��ԣ�Na2SO4��Һ������ | |
| C�� | H3PO4��Һ�ĵ�����������H2SO4��Һ | |
| D�� | H2SO4��Һ��Na3PO4��Ӧ��������H3PO4��Na2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ֻ�ѧ�� | B�� | �����ۼ������ӻ����� | ||
| C�� | ������������ | D�� | �����ۼ��Ĺ��ۻ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com